The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase
- PMID: 21752907
- PMCID: PMC3187482
- DOI: 10.1128/JVI.05265-11
The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase
Abstract
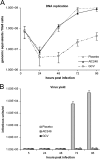
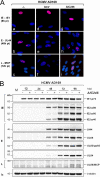
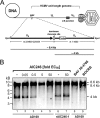
Human cytomegalovirus (HCMV) remains the leading viral cause of birth defects and life-threatening disease in transplant recipients. All approved antiviral drugs target the viral DNA polymerase and are associated with severe toxicity issues and the emergence of drug resistance. Attempts to discover improved anti-HCMV drugs led to the identification of the small-molecular-weight compound AIC246 (Letermovir). AIC246 exhibits outstanding anti-HCMV activity in vitro and in vivo and currently is undergoing a clinical phase IIb trial. The initial mode-of-action studies suggested that the drug acts late in the HCMV replication cycle via a mechanism distinct from that of polymerase inhibitors. Here, we extend our mode-of-action analyses and report that AIC246 blocks viral replication without inhibiting the synthesis of progeny HCMV DNA or viral proteins. The genotyping of mutant viruses that escaped AIC246 inhibition uncovered distinct point mutations in the UL56 subunit of the viral terminase complex. Marker transfer analyses confirmed that these mutations were sufficient to mediate AIC246 resistance. The mapping of drug resistance to open reading frame UL56 suggests that viral DNA processing and/or packaging is targeted by AIC246. In line with this, we demonstrate that AIC246 affects the formation of proper unit-length genomes from viral DNA concatemers and interferes with virion maturation. However, since AIC246-resistant viruses do not exhibit cross-resistance to previously published terminase inhibitors, our data suggest that AIC246 interferes with HCMV DNA cleavage/packaging via a molecular mechanism that is distinct from that of other compound classes known to target the viral terminase.
Figures


References
-
- Andrei G., De C. E., Snoeck R. 2009. Drug targets in cytomegalovirus infection. Infect. Disord. Drug Targets 9: 201–222 - PubMed
-
- Andreoni M., Faircloth M., Vugler L., Britt W. J. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23: 157–167 - PubMed
-
- Becke S., et al. 2010. Optimized recombinant dense bodies of human cytomegalovirus efficiently prime virus specific lymphocytes and neutralizing antibodies without the addition of adjuvant. Vaccine 28: 6191–6198 - PubMed
-
- Biswas S., Swift M., Field H. J. 2007. High frequency of spontaneous helicase-primase inhibitor (BAY 57-1293) drug-resistant variants in certain laboratory isolates of HSV-1. Antivir. Chem. Chemother. 18: 13–23 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

