The leader protein of cardioviruses inhibits stress granule assembly
- PMID: 21752908
- PMCID: PMC3165746
- DOI: 10.1128/JVI.00480-11
The leader protein of cardioviruses inhibits stress granule assembly
Abstract
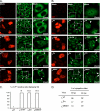
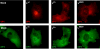
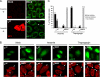
Stress granules (SG) are cytoplasmic aggregates of stalled translation preinitiation complexes that form in cells exposed to various environmental stresses. Here, we show that stress granules assemble in cells infected with Theiler's murine encephalomyelitis virus (TMEV) mutants carrying alterations in the leader (L) protein, but not in cells infected with wild-type TMEV. Stress granules also formed in STAT1-deficient cells, suggesting that SG formation was not a consequence of increased type I interferon (IFN) production when cells were infected with the mutant virus. Ectopic expression of the wild-type L protein was sufficient to inhibit stress granule formation induced by sodium arsenite or thapsigargin treatment. In conclusion, TMEV infection induces stress granule assembly, but this process is inhibited by the L protein. Unlike poliovirus-induced stress granules, TMEV-induced stress granules did not contain the nuclear protein Sam68 but contained polypyrimidine tract binding protein (PTB), an internal ribosome entry site (IRES)-interacting protein. Moreover, G3BP was not degraded and was found in SG after TMEV infection, suggesting that SG content could be virus specific. Despite the colocalization of PTB with SG and the known interaction of PTB with viral RNA, in situ hybridization and immunofluorescence assays failed to detect viral RNA trapped in infection-induced SG. Recombinant Theiler's viruses expressing the L protein of Saffold virus 2 (SAFV-2), a closely related human theilovirus, or the L protein of mengovirus, an encephalomyocarditis virus (EMCV) strain, also inhibited infection-induced stress granule assembly, suggesting that stress granule antagonism is a common feature of cardiovirus L proteins.
Figures



References
-
- Anderson P., Kedersha N. 2009. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat. Rev. Mol. Cell Biol. 10:430–436 - PubMed
-
- Anderson P., Kedersha N. 2009. Stress granules. Curr. Biol. 19:R397–R398 - PubMed
-
- Brahic M., Bureau J. F., Michiels T. 2005. The genetics of the persistent infection and demyelinating disease caused by Theiler's virus. Annu. Rev. Microbiol. 59:279–298 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

