Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output
- PMID: 21752931
- PMCID: PMC3143561
- DOI: 10.1242/dev.065797
Live imaging of the Drosophila spermatogonial stem cell niche reveals novel mechanisms regulating germline stem cell output
Abstract
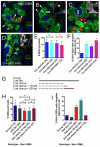
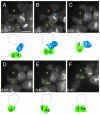
Adult stem cells modulate their output by varying between symmetric and asymmetric divisions, but have rarely been observed in living intact tissues. Germline stem cells (GSCs) in the Drosophila testis are anchored to somatic hub cells and were thought to exclusively undergo oriented asymmetric divisions, producing one stem cell that remains hub-anchored and one daughter cell displaced out of the stem cell-maintaining micro-environment (niche). We developed extended live imaging of the Drosophila testis niche, allowing us to track individual germline cells. Surprisingly, new wild-type GSCs are generated in the niche during steady-state tissue maintenance by a previously undetected event we term 'symmetric renewal', where interconnected GSC-daughter cell pairs swivel such that both cells contact the hub. We also captured GSCs undergoing direct differentiation by detaching from the hub. Following starvation-induced GSC loss, GSC numbers are restored by symmetric renewals. Furthermore, upon more severe (genetically induced) GSC loss, both symmetric renewal and de-differentiation (where interconnected spermatogonia fragment into pairs while moving towards then establishing contact with the hub) occur simultaneously to replenish the GSC pool. Thus, stereotypically oriented stem cell divisions are not always correlated with an asymmetric outcome in cell fate, and changes in stem cell output are governed by altered signals in response to tissue requirements.
Figures



References
-
- Barroca V., Lassalle B., Coureuil M., Louis J. P., Le Page F., Testart J., Allemand I., Riou L., Fouchet P. (2009). Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat. Cell Biol. 11, 190-196 - PubMed
-
- Bloor J. W., Kiehart D. P. (2001). zipper Nonmuscle myosin-II functions downstream of PS2 integrin in Drosophila myogenesis and is necessary for myofibril formation. Dev. Biol. 239, 215-228 - PubMed
-
- Boyle M., Wong C., Rocha M., Jones D. L. (2007). Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 1, 470-478 - PubMed
-
- Brawley C., Matunis E. (2004). Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304, 1331-1334 - PubMed
-
- Carpenter A. T. (1981). EM autoradiographic evidence that DNA synthesis occurs at recombination nodules during meiosis in Drosophila melanogaster females. Chromosoma 83, 59-80 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

