no child left behind encodes a novel chromatin factor required for germline stem cell maintenance in males but not females
- PMID: 21752937
- PMCID: PMC3143560
- DOI: 10.1242/dev.067942
no child left behind encodes a novel chromatin factor required for germline stem cell maintenance in males but not females
Abstract
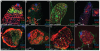
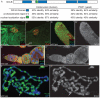
Male and female germ cells follow distinct developmental paths with respect to germline stem cell (GSC) production and the types of differentiated progeny they produce (sperm versus egg). An essential aspect of germline development is how sexual identity is used to differentially regulate the male and female germ cell genomes to allow for these distinct outcomes. Here, we identify a gene, no child left behind (nclb), that plays very different roles in the male versus female germline in Drosophila. In particular, nclb is required for GSC maintenance in males, but not in females. Male GSCs mutant for nclb are rapidly lost from the niche, and begin to differentiate but cannot complete spermatogenesis. We further find that nclb encodes a member of a new family of conserved chromatin-associated proteins. NCLB interacts with chromatin in a specific manner and is associated with sites of active transcription. Thus, NCLB appears to be a novel chromatin regulator that exhibits very different effects on the male and female germ cell genomes.
Figures




References
-
- Aboïm A. N. (1945). Développement embryonnaire et post-embryonnaire des gonades normales et agamétiques de Drosophila melanogaster. Rev. Suisse Zool. 52, 53-154
-
- Camara N., Whitworth C., Van Doren M. (2008). The creation of sexual dimorphism in the Drosophila soma. Curr. Top. Dev. Biol. 83, 65-107 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

