Performance of a rapid and simple HIV testing algorithm in a multicenter phase III microbicide clinical trial
- PMID: 21752945
- PMCID: PMC3165239
- DOI: 10.1128/CVI.05069-11
Performance of a rapid and simple HIV testing algorithm in a multicenter phase III microbicide clinical trial
Abstract
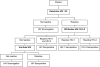
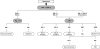
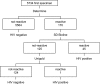
A multitest sequential algorithm based on rapid and simple (R/S) assays was applied for the diagnosis of HIV infection among participants in a phase 3 microbicide effectiveness trial. HIV testing was performed on finger-prick blood samples obtained from patients after their enrollment in the trial. The specimens were tested in a serial procedure using three different rapid tests (Determine HIV-1/2 [Abbott], SD Bioline HIV-1/2 3.0 [Standard Diagnostics], and Uni-Gold HIV [Trinity Biotech]). In the event of discordant results between the Determine HIV-1/2 and SD Bioline HIV-1/2 3.0 tests, the third assay (Uni-Gold HIV) determined the final outcome. When the final outcome was positive, a second specimen was collected and tested with the same algorithm, only if a positive result was obtained with this sample the participant was informed of her positive serostatus. A total of 5,734 postenrollment specimens obtained from 1,398 women were tested. Forty-six women tested positive according to the testing algorithm performed on the first collected specimen. Confirmatory testing results obtained at the ITM confirmed that 42 women were truly infected. Two of four initial false positives tested negative upon analysis of a second blood specimen. The other two tested false positive twice using specimens collected the same day. A high percentage of specimens reactive with the Determine HIV-1/2 assay was only observed at the study site in Kampala. This result did not appear to be associated with pregnancy or malaria infection. We conclude that HIV testing algorithms, including only R/S assays, are suitable for use in clinical trials, provided that adequate quality assurance procedures are in place.
Figures
References
-
- Association of Public Health Laboratories/Centers for Disease Control Prevention 2009. HIV testing algorithms: a status report 2009. Association of Public Health Laboratories, Silver Spring, MD: http://www.aphl.org/aphlprograms/infectious/hiv/Pages/HIVStatusReport.aspx
-
- Branson B. M. 2000. Rapid tests for HIV antibody. AIDS Rev. 2:76–83
-
- Centers for Disease Control Prevention 2004. Notice to readers: protocols for confirmation of reactive rapid HIV tests MMWR Morb. Mortal. Wkly. Rep. 53:221–222 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5310a7.htm
-
- Chen M. Y., Estcourt C. S. 2009. Time to roll out rapid testing for HIV? Yes, but with appropriate safeguards. Sex. Health 6:1–3 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

