Comparison of plasmid vaccine immunization schedules using intradermal in vivo electroporation
- PMID: 21752954
- PMCID: PMC3165233
- DOI: 10.1128/CVI.05045-11
Comparison of plasmid vaccine immunization schedules using intradermal in vivo electroporation
Abstract
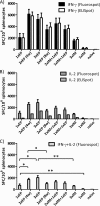
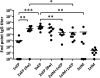
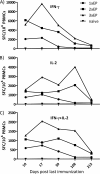
In vivo electroporation (EP) has proven to significantly increase plasmid transfection efficiency and to augment immune responses after immunization with plasmids. In this study, we attempted to establish an immunization protocol using intradermal (i.d.) EP. BALB/c mice were immunized with a plasmid encoding HIV-1 p37Gag, either i.d. with the Derma Vax EP device, intramuscularly (i.m.) without EP, or with combinations of both. A novel FluoroSpot assay was used to evaluate the vaccine-specific cellular immune responses. The study showed that i.d. EP immunizations induced stronger immune responses than i.m. immunizations using a larger amount of DNA and that repeated i.d. EP immunizations induced stronger immune responses than i.m. priming followed by i.d. EP boosting. Two and three i.d. EP immunizations induced immune responses of similar magnitude, and a short interval between immunizations was superior to a longer interval in terms of the magnitude of cellular immune responses. The FluoroSpot assay allowed for the quantification of vaccine-specific cells secreting either gamma interferon (IFN-γ), interleukin-2 (IL-2), or both, and the sensitivity of the assay was confirmed with IFN-γ and IL-2 enzyme-linked immunosorbent spot (ELISpot) assays. The data obtained in this study can aid in the design of vaccine protocols using i.d. EP, and the results emphasize the advantages of the FluoroSpot assay over traditional ELISpot assay and intracellular staining for the detection and quantification of bifunctional vaccine-specific immune responses.
Figures


References
-
- Babiuk S., Baca-Estrada M., Babiuk L. A., Ewen C., Foldvari M. 2000. Cutaneous vaccination: the skin as an immunologically active tissue and the challenge of antigen delivery. J. Control. Release 66:199–214 - PubMed
-
- Bergman P. J., et al. 2006. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine 24:4582–4585 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

