Immunosuppressive effects of streptozotocin-induced diabetes result in absolute lymphopenia and a relative increase of T regulatory cells
- PMID: 21752956
- PMCID: PMC3161310
- DOI: 10.2337/db11-0159
Immunosuppressive effects of streptozotocin-induced diabetes result in absolute lymphopenia and a relative increase of T regulatory cells
Abstract
Objective: Streptozotocin (STZ) is the most widely used diabetogenic agent in animal models of islet transplantation. However, the immunomodifying effects of STZ and the ensuing hyperglycemia on lymphocyte subsets, particularly on T regulatory cells (Tregs), remain poorly understood.
Research design and methods: This study evaluated how STZ-induced diabetes affects adaptive immunity and the consequences thereof on allograft rejection in murine models of islet and skin transplantation. The respective toxicity of STZ and hyperglycemia on lymphocyte subsets was tested in vitro. The effect of hyperglycemia was assessed independently of STZ in vivo by the removal of transplanted syngeneic islets, using an insulin pump, and with rat insulin promoter diphtheria toxin receptor transgenic mice.
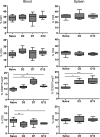
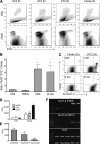
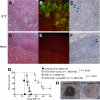
Results: Early lymphopenia in both blood and spleen was demonstrated after STZ administration. Direct toxicity of STZ on lymphocytes, particularly on CD8(+) cells and B cells, was shown in vitro. Hyperglycemia also correlated with blood and spleen lymphopenia in vivo but was not lymphotoxic in vitro. Independently of hyperglycemia, STZ led to a relative increase of Tregs in vivo, with the latter retaining their suppressive capacity in vitro. The higher frequency of Tregs was associated with Treg proliferation in the blood, but not in the spleen, and higher blood levels of transforming growth factor-β. Finally, STZ administration delayed islet and skin allograft rejection compared with naive mice.
Conclusions: These data highlight the direct and indirect immunosuppressive effects of STZ and acute hyperglycemia, respectively. Thus, these results have important implications for the future development of tolerance-based protocols and their translation from the laboratory to the clinic.
Figures



References
-
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008;51:216–226 - PubMed
-
- Gaulton GN, Schwartz JL, Eardley DD. Assessment of the diabetogenic drugs alloxan and streptozotocin as models for the study of immune defects in diabetic mice. Diabetologia 1985;28:769–775 - PubMed
-
- Nichols WK, Spellman JB, Vann LL, Daynes RA. Immune responses of diabetic animals: direct immunosuppressant effects of streptozotocin in mice. Diabetologia 1979;16:51–57 - PubMed
-
- Takayama Y, Ichikawa T, Maki T. Effect of STZ administration on islet isograft and allograft survival in NOD mice. Diabetes 1993;42:324–329 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

