Cyclosporin A inhibits the replication of diverse coronaviruses
- PMID: 21752960
- PMCID: PMC3352363
- DOI: 10.1099/vir.0.034983-0
Cyclosporin A inhibits the replication of diverse coronaviruses
Abstract
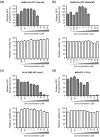
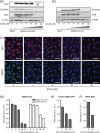
Low micromolar, non-cytotoxic concentrations of cyclosporin A (CsA) strongly affected the replication of severe acute respiratory syndrome coronavirus (SARS-CoV), human coronavirus 229E and mouse hepatitis virus in cell culture, as was evident from the strong inhibition of GFP reporter gene expression and a reduction of up to 4 logs in progeny titres. Upon high-multiplicity infection, CsA treatment rendered SARS-CoV RNA and protein synthesis almost undetectable, suggesting an early block in replication. siRNA-mediated knockdown of the expression of the prominent CsA targets cyclophilin A and B did not affect SARS-CoV replication, suggesting either that these specific cyclophilin family members are dispensable or that the reduced expression levels suffice to support replication.
Figures
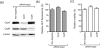
References
-
- Cervantes-Barragan L., Züst R., Maier R., Sierro S., Janda J., Levy F., Speiser D., Romero P., Rohrlich P. S., et al. (2010). Dendritic cell-specific antigen delivery by coronavirus vaccine vectors induces long-lasting protective antiviral and antitumor immunity. MBio 1, e00171–e00110 10.1128/mBio.00171-10 - DOI - PMC - PubMed
-
- Chatterji U., Lim P., Bobardt M. D., Wieland S., Cordek D. G., Vuagniaux G., Chisari F., Cameron C. E., Targett-Adams P., et al. (2010). HCV resistance to cyclosporin A does not correlate with a resistance of the NS5A-cyclophilin A interaction to cyclophilin inhibitors. J Hepatol 53, 50–56 10.1016/j.jhep.2010.01.041 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

