βCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting αCaMKII to synapses
- PMID: 21752990
- PMCID: PMC6623068
- DOI: 10.1523/JNEUROSCI.5105-10.2011
βCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting αCaMKII to synapses
Abstract
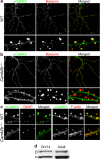
The calcium/calmodulin-dependent kinase type II (CaMKII) holoenzyme of the forebrain predominantly consists of heteromeric complexes of the αCaMKII and βCaMKII isoforms. Yet, in contrast to αCaMKII, the role of βCaMKII in hippocampal synaptic plasticity and learning has not been investigated. Here, we compare two targeted Camk2b mouse mutants to study the role of βCaMKII in hippocampal function. Using a Camk2b(-/-) mutant, in which βCaMKII is absent, we show that both hippocampal-dependent learning and Schaffer collateral-CA1 long-term potentiation (LTP) are highly dependent upon the presence of βCaMKII. We further show that βCaMKII is required for proper targeting of αCaMKII to the synapse, indicating that βCaMKII regulates the distribution of αCaMKII between the synaptic pool and the adjacent dendritic shaft. In contrast, localization of αCaMKII, hippocampal synaptic plasticity and learning were unaffected in the Camk2b(A303R) mutant, in which the calcium/calmodulin-dependent activation of βCaMKII is prevented, while the F-actin binding and bundling property is preserved. This indicates that the calcium/calmodulin-dependent kinase activity of βCaMKII is fully dispensable for hippocampal learning, LTP, and targeting of αCaMKII, but implies a critical role for the F-actin binding and bundling properties of βCaMKII in synaptic function. Together, our data provide compelling support for a model of CaMKII function in which αCaMKII and βCaMKII act in concert, but with distinct functions, to regulate hippocampal synaptic plasticity and learning.
Figures





References
-
- Bayer KU, Löhler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70:147–154. - PubMed
-
- Brocke L, Chiang LW, Wagner PD, Schulman H. Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem. 1999;274:22713–22722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
