Bcl11b/Ctip2 controls the differentiation of vomeronasal sensory neurons in mice
- PMID: 21752992
- PMCID: PMC3394408
- DOI: 10.1523/JNEUROSCI.1245-11.2011
Bcl11b/Ctip2 controls the differentiation of vomeronasal sensory neurons in mice
Abstract
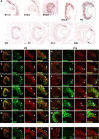
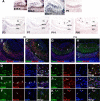
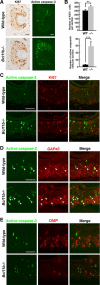
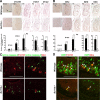
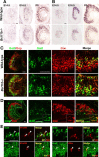
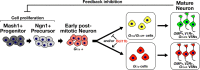
The transcription factor Bcl11b/Ctip2 plays critical roles in the development of several systems and organs, including the immune system, CNS, skin, and teeth. Here, we show that Bcl11b/Ctip2 is highly expressed in the developing vomeronasal system in mice and is required for its proper development. Bcl11b/Ctip2 is expressed in postmitotic vomeronasal sensory neurons (VSNs) in the vomeronasal epithelium (VNE) as well as projection neurons and GABAergic interneurons in the accessory olfactory bulb (AOB). In the absence of Bcl11b, these neurons are born in the correct number, but VSNs selectively die by apoptosis. The critical role of Bcl11b in vomeronasal system development is demonstrated by the abnormal phenotypes of Bcl11b-deficient mice: disorganization of layer formation of the AOB, impaired axonal projections of VSNs, a significant reduction in the expression of vomeronasal receptor genes, and defective mature differentiation of VSNs. VSNs can be classified into two major types of neurons, vomeronasal 1 receptor (V1r)/Gα(i2)-positive and vomeronasal 2 receptor (V2r)/Gα(o)-positive VSNs. We found that all Gα(i2)-positive cells coexpressed Gα(o) during embryogenesis. This coexpression is also observed in newly differentiated neurons in the adult VNE. Interestingly, loss of Bcl11b function resulted in an increased number of V1r/Gα(i2)-type VSNs and a decreased number of V2r/Gα(o)-type VSNs, suggesting that Bcl11b regulates the fate choice between these two VSN types. These results indicate that Bcl11b/Ctip2 is an essential regulator of the differentiation and dichotomy of VSNs.
Figures




Comment in
-
Molecular switches in the development and fate specification of vomeronasal neurons.J Neurosci. 2011 Dec 7;31(49):17761-3. doi: 10.1523/JNEUROSCI.4682-11.2011. J Neurosci. 2011. PMID: 22159092 Free PMC article. No abstract available.
References
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Avram D, Fields A, Pretty On Top K, Nevrivy DJ, Ishmael JE, Leid M. Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J Biol Chem. 2000;275:10315–10322. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
