Age-related decline in circadian output
- PMID: 21752996
- PMCID: PMC3155746
- DOI: 10.1523/JNEUROSCI.0451-11.2011
Age-related decline in circadian output
Abstract
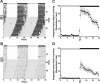
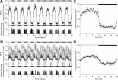
Disruptions in sleep/wake cycles, including decreased amplitude of rhythmic behaviors and fragmentation of the sleep episodes, are commonly associated with aging in humans and other mammals. While there are undoubtedly many factors contributing to these changes, a body of literature is emerging, suggesting that an age-related decline in the central circadian clock in the suprachiasmatic nucleus (SCN) may be a key element responsible. To explore age-related changes in the SCN, we have performed in vivo multiunit neural activity (MUA) recordings from the SCN of freely moving young (3-5 months) and middle-aged (13-18 months) mice. Importantly, the amplitude of day-night difference in MUA was significantly reduced in the older mice. We also found that the neural activity rhythms are clearly degraded in the subparaventricular zone, one of the main neural outputs of the SCN. Surprisingly, parallel studies indicate that the molecular clockwork in the SCN as measured by PER2 exhibited only minor deficits at this same age. Thus, the circadian output measured at the level of neural activity rhythms in the SCN is degraded by aging, and this decline occurs before the disruption of key components of the molecular clockwork.
Figures


References
-
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res. 2001;66:1133–1139. - PubMed
-
- Aujard F, Herzog ED, Block GD. Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neurosci. 2001;106:255–261. - PubMed
-
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
