Coding of stereoscopic depth information in visual areas V3 and V3A
- PMID: 21753004
- PMCID: PMC3143190
- DOI: 10.1523/JNEUROSCI.5956-10.2011
Coding of stereoscopic depth information in visual areas V3 and V3A
Abstract
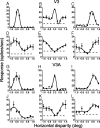
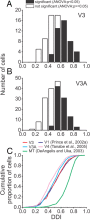
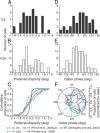
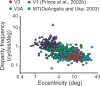
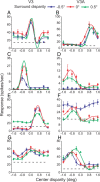
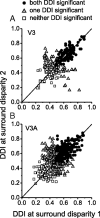
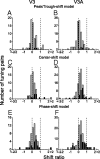
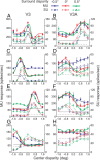
The process of stereoscopic depth perception is thought to begin with the analysis of absolute binocular disparity, the difference in position of corresponding features in the left and right eye images with respect to the points of fixation. Our sensitivity to depth, however, is greater when depth judgments are based on relative disparity, the difference between two absolute disparities, compared to when they are based on absolute disparity. Therefore, the visual system is thought to compute relative disparities for fine depth discrimination. Functional magnetic resonance imaging studies in humans and monkeys have suggested that visual areas V3 and V3A may be specialized for stereoscopic depth processing based on relative disparities. In this study, we measured absolute and relative disparity-tuning of neurons in V3 and V3A of alert fixating monkeys, and we compared their basic tuning properties with those published previously for other visual areas. We found that neurons in V3 and V3A predominantly encode absolute, not relative, disparities. We also found that basic parameters of disparity-tuning in V3 and V3A are similar to those from other extrastriate visual areas. Finally, by comparing single-unit activity with multi-unit activity measured at the same recording site, we demonstrate that neurons with similar disparity selectivity are clustered in both V3 and V3A. We conclude that areas V3 and V3A are not particularly specialized for processing stereoscopic depth information compared to other early visual areas, at least with respect to the tuning properties that we have examined.
Figures


References
-
- Adams DL, Zeki S. Functional organization of macaque V3 for stereoscopic depth. J Neurophysiol. 2001;86:2195–2203. - PubMed
-
- Backus BT, Fleet DJ, Parker AJ, Heeger DJ. Human cortical activity correlates with stereoscopic depth perception. J Neurophysiol. 2001;86:2054–2068. - PubMed
-
- Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991;353:429–431. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
