Animal escapology II: escape trajectory case studies
- PMID: 21753040
- PMCID: PMC3135389
- DOI: 10.1242/jeb.053801
Animal escapology II: escape trajectory case studies
Abstract
Escape trajectories (ETs; measured as the angle relative to the direction of the threat) have been studied in many taxa using a variety of methodologies and definitions. Here, we provide a review of methodological issues followed by a survey of ET studies across animal taxa, including insects, crustaceans, molluscs, lizards, fish, amphibians, birds and mammals. Variability in ETs is examined in terms of ecological significance and morpho-physiological constraints. The survey shows that certain escape strategies (single ETs and highly variable ETs within a limited angular sector) are found in most taxa reviewed here, suggesting that at least some of these ET distributions are the result of convergent evolution. High variability in ETs is found to be associated with multiple preferred trajectories in species from all taxa, and is suggested to provide unpredictability in the escape response. Random ETs are relatively rare and may be related to constraints in the manoeuvrability of the prey. Similarly, reports of the effect of refuges in the immediate environment are relatively uncommon, and mainly confined to lizards and mammals. This may be related to the fact that work on ETs carried out in laboratory settings has rarely provided shelters. Although there are a relatively large number of examples in the literature that suggest trends in the distribution of ETs, our understanding of animal escape strategies would benefit from a standardization of the analytical approach in the study of ETs, using circular statistics and related tests, in addition to the generation of large data sets.
Figures



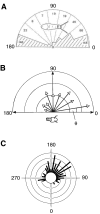





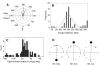


References
-
- Ansell A. D., Cattaneo-Vietti R., Chiantore M. (1998). Swimming in the Antarctic scallop Adamussium colbecki: analysis of in situ video recordings. Antarct. Sci. 10, 369-375
-
- Arnott S. A., Neil D. M., Ansell A. D. (1999). Escape trajectories of the brown shrimp Crangon crangon, and a theoretical consideration of initial escape angles from predators. J. Exp. Biol. 202, 193-209 - PubMed
-
- Azizi E., Landberg T. (2002). Effects of metamorphosis on the aquatic escape response of the two-lined salamander (Eurycea bislineata). J. Exp. Biol. 205, 841-849 - PubMed
-
- Bailey D. M., Johnston I. A. (2005). Scallop swimming kinematics and muscle performance: modelling the effects of “within-animal” variation in temperature sensitivity. Mar. Freshw. Behav. Physiol. 38, 1-19
-
- Batschelet E. (1981). Circular Statistics in Biology. New York: Academic Press;
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

