ENaC structure and function in the wake of a resolved structure of a family member
- PMID: 21753073
- PMCID: PMC3191808
- DOI: 10.1152/ajprenal.00259.2011
ENaC structure and function in the wake of a resolved structure of a family member
Abstract
Our understanding of epithelial Na(+) channel (ENaC) structure and function has been profoundly impacted by the resolved structure of the homologous acid-sensing ion channel 1 (ASIC1). The structure of the extracellular and pore regions provide insight into channel assembly, processing, and the ability of these channels to sense the external environment. The absence of intracellular structures precludes insight into important interactions with intracellular factors that regulate trafficking and function. The primary sequences of ASIC1 and ENaC subunits are well conserved within the regions that are within or in close proximity to the plasma membrane, but poorly conserved in peripheral domains that may functionally differentiate family members. This review examines functional data, including ion selectivity, gating, and amiloride block, in light of the resolved ASIC1 structure.
Figures


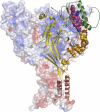
References
-
- Adachi M, Kitamura K, Miyoshi T, Narikiyo T, Iwashita K, Shiraishi N, Nonoguchi H, Tomita K. Activation of epithelial sodium channels by prostasin in Xenopus oocytes. J Am Soc Nephrol 12: 1114–1121, 2001 - PubMed
-
- Adams CM, Snyder PM, Welsh MJ. Paradoxical stimulation of a DEG/ENaC channel by amiloride. J Biol Chem 274: 15500–15504, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

