Ectopic expression of AtJMT in Nicotiana attenuata: creating a metabolic sink has tissue-specific consequences for the jasmonate metabolic network and silences downstream gene expression
- PMID: 21753114
- PMCID: PMC3165883
- DOI: 10.1104/pp.111.178582
Ectopic expression of AtJMT in Nicotiana attenuata: creating a metabolic sink has tissue-specific consequences for the jasmonate metabolic network and silences downstream gene expression
Abstract
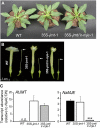
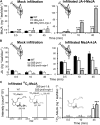
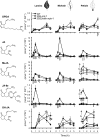
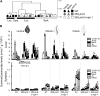
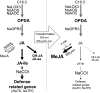
To create a metabolic sink in the jasmonic acid (JA) pathway, we generated transgenic Nicotiana attenuata lines ectopically expressing Arabidopsis (Arabidopsis thaliana) jasmonic acid O-methyltransferase (35S-jmt) and additionally silenced in other lines the N. attenuata methyl jasmonate esterase (35S-jmt/ir-mje) to reduce the deesterification of methyl jasmonate (MeJA). Basal jasmonate levels did not differ between transgenic and wild-type plants; however, after wounding and elicitation with Manduca sexta oral secretions, the bursts of JA, jasmonoyl-isoleucine (JA-Ile), and their metabolites that are normally observed in the lamina, midvein, and petiole of elicited wild-type leaves were largely absent in both transformants but replaced by a burst of endogenous MeJA that accounted for almost half of the total elicited jasmonate pools. In these plants, MeJA became a metabolic sink that affected the jasmonate metabolic network and its spread to systemic leaves, with major effects on 12-oxo-phytodieonic acid, JA, and hydroxy-JA in petioles and on JA-Ile in laminas. Alterations in the size of jasmonate pools were most obvious in systemic tissues, especially petioles. Expression of threonine deaminase and trypsin proteinase inhibitor, two JA-inducible defense genes, was strongly decreased in both transgenic lines without influencing the expression of JA biosynthesis genes that were uncoupled from the wounding and elicitation with M. sexta oral secretions-elicited JA-Ile gradient in elicited leaves. Taken together, this study provides support for a central role of the vasculature in the propagation of jasmonates and new insights into the versatile spatiotemporal characteristics of the jasmonate metabolic network.
Figures



Similar articles
-
RuBPCase activase (RCA) mediates growth-defense trade-offs: silencing RCA redirects jasmonic acid (JA) flux from JA-isoleucine to methyl jasmonate (MeJA) to attenuate induced defense responses in Nicotiana attenuata.New Phytol. 2014 Mar;201(4):1385-1395. doi: 10.1111/nph.12591. Epub 2013 Nov 11. New Phytol. 2014. PMID: 24491116 Free PMC article.
-
Diverting the flux of the JA pathway in Nicotiana attenuata compromises the plant's defense metabolism and fitness in nature and glasshouse.PLoS One. 2011;6(10):e25925. doi: 10.1371/journal.pone.0025925. Epub 2011 Oct 10. PLoS One. 2011. PMID: 22022469 Free PMC article.
-
Methyl jasmonate-elicited herbivore resistance: does MeJA function as a signal without being hydrolyzed to JA?Planta. 2008 Apr;227(5):1161-8. doi: 10.1007/s00425-008-0690-8. Epub 2008 Jan 23. Planta. 2008. PMID: 18214527 Free PMC article.
-
The essential role of jasmonic acid in plant-herbivore interactions--using the wild tobacco Nicotiana attenuata as a model.J Genet Genomics. 2013 Dec 20;40(12):597-606. doi: 10.1016/j.jgg.2013.10.001. Epub 2013 Nov 9. J Genet Genomics. 2013. PMID: 24377866 Review.
-
Using natural variation to achieve a whole-plant functional understanding of the responses mediated by jasmonate signaling.Plant J. 2019 Aug;99(3):414-425. doi: 10.1111/tpj.14331. Epub 2019 Apr 29. Plant J. 2019. PMID: 30927293 Review.
Cited by
-
Identification of a novel jasmonate-responsive element in the AtJMT promoter and its binding protein for AtJMT repression.PLoS One. 2013;8(2):e55482. doi: 10.1371/journal.pone.0055482. Epub 2013 Feb 5. PLoS One. 2013. Retraction in: PLoS One. 2020 Nov 3;15(11):e0242041. doi: 10.1371/journal.pone.0242041. PMID: 23393583 Free PMC article. Retracted.
-
Physiological Basis and Transcriptional Profiling of Three Salt-Tolerant Mutant Lines of Rice.Front Plant Sci. 2016 Sep 28;7:1462. doi: 10.3389/fpls.2016.01462. eCollection 2016. Front Plant Sci. 2016. PMID: 27733859 Free PMC article.
-
Distinct Signatures of Host Defense Suppression by Plant-Feeding Mites.Int J Mol Sci. 2018 Oct 20;19(10):3265. doi: 10.3390/ijms19103265. Int J Mol Sci. 2018. PMID: 30347842 Free PMC article.
-
Argonaute4 Modulates Resistance to Fusarium brachygibbosum Infection by Regulating Jasmonic Acid Signaling.Plant Physiol. 2020 Oct;184(2):1128-1152. doi: 10.1104/pp.20.00171. Epub 2020 Jul 28. Plant Physiol. 2020. PMID: 32723807 Free PMC article.
-
Silencing JA hydroxylases in Nicotiana attenuata enhances jasmonic acid-isoleucine-mediated defenses against Spodoptera litura.Plant Divers. 2020 Jan 3;42(2):111-119. doi: 10.1016/j.pld.2019.11.005. eCollection 2020 Apr. Plant Divers. 2020. PMID: 32373769 Free PMC article.
References
-
- Allmann S, Baldwin IT. (2010) Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 329: 1075–1078 - PubMed
-
- Baldwin IT, Schmelz EA, Ohnmeiss TE. (1994) Wound-induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris. J Chem Ecol 20: 2139–2157 - PubMed
-
- Browse J. (2005) Jasmonate: an oxylipin signal with many roles in plants. Vitam Horm 72: 431–456 - PubMed
-
- Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, et al. (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

