Collective synthesis of natural products by means of organocascade catalysis
- PMID: 21753848
- PMCID: PMC3439143
- DOI: 10.1038/nature10232
Collective synthesis of natural products by means of organocascade catalysis
Abstract
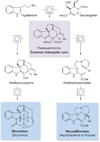
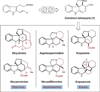
Organic chemists are now able to synthesize small quantities of almost any known natural product, given sufficient time, resources and effort. However, translation of the academic successes in total synthesis to the large-scale construction of complex natural products and the development of large collections of biologically relevant molecules present significant challenges to synthetic chemists. Here we show that the application of two nature-inspired techniques, namely organocascade catalysis and collective natural product synthesis, can facilitate the preparation of useful quantities of a range of structurally diverse natural products from a common molecular scaffold. The power of this concept has been demonstrated through the expedient, asymmetric total syntheses of six well-known alkaloid natural products: strychnine, aspidospermidine, vincadifformine, akuammicine, kopsanone and kopsinine.
©2011 Macmillan Publishers Limited. All rights reserved
Figures

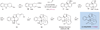


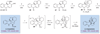
References
-
- Walji A, MacMillan DWC. Strategies to bypass the Taxol problem. Enantioselective cascade catalysis, a new approach for the efficient construction of molecular complexity. Synlett. 2007:1477–1489.
-
- Huang Y, Walji AM, Larsen CH, MacMillan DWC. Enantioselective organocascade catalysis. J. Am. Chem. Soc. 2005;127:15051–15053. - PubMed
-
- Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. 3rd edn. Wiley; 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

