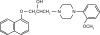Naftopidil for the treatment of urinary symptoms in patients with benign prostatic hyperplasia
- PMID: 21753885
- PMCID: PMC3132093
- DOI: 10.2147/TCRM.S13883
Naftopidil for the treatment of urinary symptoms in patients with benign prostatic hyperplasia
Abstract
Naftopidil, approved only in Japan, is an α1-adrenergic receptor antagonist (α1-blocker) used to treat lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH). Different from tamsulosin hydrochloride and silodosin, in that it has higher and extremely higher affinity respectively, for the α1A-adrenergic receptor subtype than for the α1D type, naftopidil has distinct characteristics because it has a three times greater affinity for the α1D-adrenergic receptor subtype than for the α1A subtype. Although well-designed large-scale randomized controlled studies are lacking and the optimal dosage of naftopidil is not always completely determined, previous reports from Japan have shown that naftopidil has superior efficacy to a placebo and comparable efficacy to other α1-blockers such as tamsulosin. On the other hand, the incidences of ejaculatory disorders and intraoperative floppy iris syndrome induced by naftopidil may be lower than for tamsulosin and silodosin having high affinity for the α1A-adrenergic receptor subtype. However, it remains unknown if the efficacy and safety of naftopidil in Japanese is applicable to white, black and Hispanic men having LUTS/BPH in western countries.
Keywords: benign prostatic hyperplasia; lower urinary tract symptoms; naftopidil; α1-blocker.
Figures
References
-
- Abrams P, D’Ancona C, Griffiths S, et al. Lower urinary tract symptom: etiology, patient assessment and predicting outcome from therapy. In: McConnell J, Abrams P, Denis L, Khoury S, Roehrborn C, editors. Male Lower Urinary Tract Dysfunction Evaluation and Management. Ed 21. Paris: Health Publications; 2006. pp. 69–142.
-
- Chapple C, Artibani W, Berges R, et al. New medical developments in the management of LUTS in adult men. In: McConnell J, Abrams P, Denis L, Khoury S, Roehrborn C, editors. Male Lower Urinary Tract Dysfunction Evaluation and Management. Ed 21. Paris: Health Publications; 2006. pp. 143–194.
-
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349(25):2387–2398. - PubMed
-
- Roehrborn CG, Siami P, Barkin J, et al. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol. 2008;179(2):616–621. - PubMed
-
- Roehrborn CG, Siami P, Barkin J, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 2-year results from the CombAT study. Eur Urol. 2010;57(1):123–131. - PubMed
LinkOut - more resources
Full Text Sources