3-Acetyl-5-hy-droxy-2-methyl-anthra[1,2-b]furan-6,11-dione
- PMID: 21754452
- PMCID: PMC3089359
- DOI: 10.1107/S1600536811013389
3-Acetyl-5-hy-droxy-2-methyl-anthra[1,2-b]furan-6,11-dione
Abstract
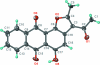
The asymmetric unit of the title compound, C(19)H(12)O(5), contains two independent mol-ecules, both slightly buckled along an axis passing through the C=O bonds of the anthraquinone ring system (r.m.s. deviation of non-H atoms = 0.082 and 0.148 Å): the benzene rings are twisted to each other by 4.3 (3)°in one mol-ecule and 10.6(3)° in the other. In both mol-ecules, the hy-droxy group forms an intra-molecular O-H⋯O hydrogen bond. The two independent mol-ecules inter-act by π-π stacking with a centroid-centroid distance of 3.539 (2) Å between hy-droxy-benzene rings of adjacent mol-ecules.
Figures


References
-
- Agilent (2010). CrysAlis PRO Agilent Technologies, Yarnton, England.
-
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
-
- Boddy, I. K., Cambie, R. C., Rutledge, P. S. & Woodgate, P. D. (1986). Aust. J. Chem. 39, 2075–2088.
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
-
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
