1-Benzyl-3,5-bis-(4-chloro-benzyl-idene)piperidin-4-one
- PMID: 21754872
- PMCID: PMC3120573
- DOI: 10.1107/S1600536811018587
1-Benzyl-3,5-bis-(4-chloro-benzyl-idene)piperidin-4-one
Abstract
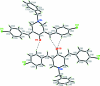
The title compound, C(26)H(21)Cl(2)NO, crystallizes with two symmetry-independent mol-ecules (A and B) in the asymmetric unit. In both mol-ecules, the central heterocyclic ring adopts a sofa conformation. The dihedral angles between the planar part of this central heterocyclic ring [maximum deviations of 0.011 (1) and 0.036 (1) Å in mol-ecules A and B, respectively] and the two almost planar [maximum deviations of 0.020 (1) and 0.008 (1) Å in A and 0.007 (1) and 0.011 (1) in B] side-chain fragments that include the aromatic ring and bridging atoms are 20.1 (1) and 31.2 (1)° in mol-ecule A, and 26.4 (1) and 19.6 (1)° in mol-ecule B. The dihedral angles between the planar part of the heterocyclic ring and the benzyl substituent are 79.7 (1) and 53.2 (1)° in mol-ecules A and B, respectively. In the crystal, weak inter-molecular C-H⋯O hydrogen bonds link the two independent mol-ecules into dimers.
Figures

References
-
- Bruker (2001). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Desiraju, G. R. & Steiner, T. (1999). The Weak Hydrogen Bond Oxford University Press.
-
- Dimmock, J. R., Padmanilayam, M. P., Puthucode, R. N., Nazarali, A. J., Motaganahalli, N. L., Zell, G. A., Quail, J. W., Oloo, E. O., Kraatz, H. B., Prisciak, J. S., Allen, T. M., Santos, C. L., Balzarini, J., De Clercq, E. & Manavathu, E. K. (2001). J. Med. Chem. 44, 586–593. - PubMed
-
- Jia, Z., Quail, J. W., Arora, V. K. & Dimmock, J. R. (1988). Acta Cryst. C44, 2114–2117.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
