SMA CARNIVAL TRIAL PART II: a prospective, single-armed trial of L-carnitine and valproic acid in ambulatory children with spinal muscular atrophy
- PMID: 21754985
- PMCID: PMC3130730
- DOI: 10.1371/journal.pone.0021296
SMA CARNIVAL TRIAL PART II: a prospective, single-armed trial of L-carnitine and valproic acid in ambulatory children with spinal muscular atrophy
Abstract
Background: Multiple lines of evidence have suggested that valproic acid (VPA) might benefit patients with spinal muscular atrophy (SMA). The SMA CARNIVAL TRIAL was a two part prospective trial to evaluate oral VPA and L-carnitine in SMA children. Part 1 targeted non-ambulatory children ages 2-8 in a 12 month cross over design. We report here Part 2, a twelve month prospective, open-label trial of VPA and L-carnitine in ambulatory SMA children.
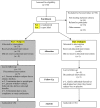
Methods: This study involved 33 genetically proven type 3 SMA subjects ages 3-17 years. Subjects underwent two baseline assessments over 4-6 weeks and then were placed on VPA and L-carnitine for 12 months. Assessments were performed at baseline, 3, 6 and 12 months. Primary outcomes included safety, adverse events and the change at 6 and 12 months in motor function assessed using the Modified Hammersmith Functional Motor Scale Extend (MHFMS-Extend), timed motor tests and fine motor modules. Secondary outcomes included changes in ulnar compound muscle action potential amplitudes (CMAP), handheld dynamometry, pulmonary function, and Pediatric Quality of Life Inventory scores.
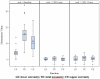
Results: Twenty-eight subjects completed the study. VPA and carnitine were generally well tolerated. Although adverse events occurred in 85% of subjects, they were usually mild and transient. Weight gain of 20% above body weight occurred in 17% of subjects. There was no significant change in any primary outcome at six or 12 months. Some pulmonary function measures showed improvement at one year as expected with normal growth. CMAP significantly improved suggesting a modest biologic effect not clinically meaningful.
Conclusions: This study, coupled with the CARNIVAL Part 1 study, indicate that VPA is not effective in improving strength or function in SMA children. The outcomes used in this study are feasible and reliable, and can be employed in future trials in SMA. TRIAL REGSITRATION: Clinicaltrials.gov NCT00227266.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

