Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses
- PMID: 21755525
- PMCID: PMC3259006
- DOI: 10.1002/mas.20342
Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses
Abstract
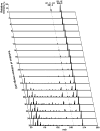
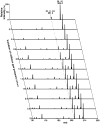
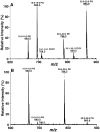
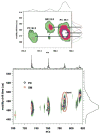
Since our last comprehensive review on multi-dimensional mass spectrometry-based shotgun lipidomics (Mass Spectrom. Rev. 24 (2005), 367), many new developments in the field of lipidomics have occurred. These developments include new strategies and refinements for shotgun lipidomic approaches that use direct infusion, including novel fragmentation strategies, identification of multiple new informative dimensions for mass spectrometric interrogation, and the development of new bioinformatic approaches for enhanced identification and quantitation of the individual molecular constituents that comprise each cell's lipidome. Concurrently, advances in liquid chromatography-based platforms and novel strategies for quantitative matrix-assisted laser desorption/ionization mass spectrometry for lipidomic analyses have been developed. Through the synergistic use of this repertoire of new mass spectrometric approaches, the power and scope of lipidomics has been greatly expanded to accelerate progress toward the comprehensive understanding of the pleiotropic roles of lipids in biological systems.
Copyright © 2011 Wiley Periodicals, Inc.
Figures








References
-
- Abe T, Norton WT. The characterization of sphingolipids from neurons and astroglia of immature rat brain. J Neurochem. 1974;23:1025–1036. - PubMed
-
- Al-Saad KA, Zabrouskov V, Siems WF, Knowles NR, Hannan RM, Hill HH., Jr Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of lipids: ionization and prompt fragmentation patterns. Rapid Commun Mass Spectrom. 2003;17:87–96. - PubMed
-
- Altelaar AF, Klinkert I, Jalink K, de Lange RP, Adan RA, Heeren RM, Piersma SR. Gold-enhanced biomolecular surface imaging of cells and tissue by SIMS and MALDI mass spectrometry. Anal Chem. 2006;78:734–742. - PubMed
-
- Andreyev AY, Fahy E, Guan Z, Kelly S, Li X, McDonald JG, Milne S, Myers D, Park H, Ryan A, Thompson BM, Wang E, Zhao Y, Brown HA, Merrill AH, Raetz CR, Russell DW, Subramaniam S, Dennis EA. Subcellular organelle lipidomics in TLR-4-activated macrophages. J Lipid Res. 2010;51:2785–2797. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

