Structure-activity relationships of antitubercular nitroimidazoles. 3. Exploration of the linker and lipophilic tail of ((s)-2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazin-6-yl)-(4-trifluoromethoxybenzyl)amine (6-amino PA-824)
- PMID: 21755942
- PMCID: PMC3158291
- DOI: 10.1021/jm1010644
Structure-activity relationships of antitubercular nitroimidazoles. 3. Exploration of the linker and lipophilic tail of ((s)-2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazin-6-yl)-(4-trifluoromethoxybenzyl)amine (6-amino PA-824)
Abstract
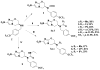
The (S)-2-nitro-6-(4-(trifluoromethoxy)benzyloxy)-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine named PA-824 (1) has demonstrated antitubercular activity in vitro and in animal models and is currently in clinical trials. We synthesized derivatives at three positions of the 4-(trifluoromethoxy)benzylamino tail, and these were tested for whole-cell activity against both replicating and nonreplicating Mycobacterium tuberculosis (Mtb). In addition, we determined their kinetic parameters as substrates of the deazaflavin-dependent nitroreductase (Ddn) from Mtb that reductively activates these pro-drugs. These studies yielded multiple compounds with 40 nM aerobic whole cell activity and 1.6 μM anaerobic whole cell activity: 10-fold improvements over both characteristics from the parent molecule. Some of these compounds exhibited enhanced solubility with acceptable stability to microsomal and in vivo metabolism. Analysis of the conformational preferences of these analogues using quantum chemistry suggests a preference for a pseudoequatorial orientation of the linker and lipophilic tail.
© 2011 American Chemical Society
Figures

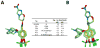




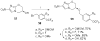
References
-
- Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, Anderson SW, Towell JA, Yuan Y, McMurray DN, Kreiswirth BN, Barry CE, Baker WR. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–966. - PubMed
-
- Sasaki H, Haraguchi Y, Itotani M, Kuroda H, Hashizume H, Tomishige T, Kawasaki M, Matsumoto M, Komatsu M, Tsubouchi H. Synthesis and antituberculosis activity of a novel series of optically active 6-nitro-2,3-dihydroimidazo[2,1-b]oxazoles. J Med Chem. 2006;49:7854–7860. - PubMed
-
- Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, Donald PR, van Niekerk C, Whitney K, Rouse DJ, Laurenzi MW, Ginsberg AM, Spigelman MK. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother. 2010;54:3402–3407. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

