Fluorescent DNA nanotags featuring covalently attached intercalating dyes: synthesis, antibody conjugation, and intracellular imaging
- PMID: 21755981
- PMCID: PMC3163149
- DOI: 10.1021/bc100485f
Fluorescent DNA nanotags featuring covalently attached intercalating dyes: synthesis, antibody conjugation, and intracellular imaging
Abstract
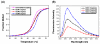
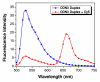
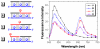
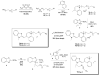
We have synthesized fluorescent DNA duplexes featuring multiple thiazole orange (TO) intercalating dyes covalently attached to the DNA via a triazole linkage. The intercalating dyes stabilize the duplex against thermal denaturation and show bright fluorescence in the green region of the spectrum. The emission color can be changed to orange or red by addition of energy-accepting Cy3 or Cy5 dyes attached covalently to the DNA duplex. The dye-modified DNA duplexes were then attached to a secondary antibody for intracellular fluorescence imaging of centrosomes in Drosophila embryos. Bright fluorescent foci were observed at the centrosomes in both the donor (TO) and acceptor (Cy5) channels, because the energy transfer efficiency is moderate. Monitoring the Cy5 emission channel significantly minimized the background signal because of the large shift in emission wavelength allowed by energy transfer.
Figures



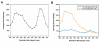
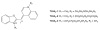
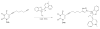
References
-
- Waggoner A. Fluorescent Labels for Proteomics and Genomics. Curr. Opin. Chem. Biol. 2006;10:62–66. - PubMed
-
- Giepmans BNG, Adams SR, Ellisman MH, Tsien RY. The Fluorescent Toolbox for Assessing Protein Location and Function. Science. 2006;312:217–224. - PubMed
-
- Bruchez MJ, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science. 1998;281:2013–2016. - PubMed
-
- Zhao X, Bagwe RP, Tan W. Development of Organic-Dye-Doped Silica Nanoparticles in a Reverse Microemulsion. Adv. Mater. 2004;16:173–176.
-
- Burns A, Ow H, Wiesner U. Fluorescent Core=Shell Silica Nanoparticles: Towards “Lab on a Particle” Architectures for Nanobiotechnology. Chem. Soc. Rev. 2006;35:1028–1042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

