Multivalent cyclic RGD conjugates for targeted delivery of small interfering RNA
- PMID: 21755983
- PMCID: PMC3718025
- DOI: 10.1021/bc200235q
Multivalent cyclic RGD conjugates for targeted delivery of small interfering RNA
Abstract
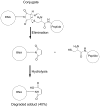
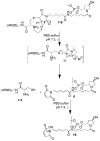
We have designed, synthesized, and tested conjugates of chemically modified luciferase siRNA (Luc-siRNA) with bi-, tri-, and tetravalent cyclic(arginine-glycine-aspartic) (cRGD) peptides that selectively bind to the αvβ3 integrin. The cellular uptake, subcellular distribution, and pharmacological effects of the cRGD-conjugated Luc-siRNAs compared to those of unconjugated controls were examined using a luciferase reporter cassette stably transfected into αvβ3 positive M21(+) human melanoma cells. The M21(+) cells exhibited receptor-mediated uptake of cRGD-siRNA conjugates but not of unconjugated control siRNA. The fluorophore-tagged cRGD-siRNA conjugates were taken up by a caveolar endocytotic route and primarily accumulated in cytosolic vesicles. The bi-, tri-, and tetravalent cRGD conjugates were taken up by M21(+) cells to approximately the same degree. However, there were notable differences in their pharmacological effectiveness. The tri- and tetravalent versions produced progressive, dose-dependent reductions in the level of luciferase expression, while the bivalent version had little effect. The basis for this divergence of uptake and effect is currently unclear. Nonetheless, the high selectivity and substantial "knock down" effects of the multivalent cRGD-siRNA conjugates suggest that this targeting and delivery strategy deserves further exploration.
Figures



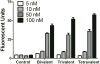
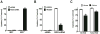



References
-
- Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–93. - PubMed
-
- Prakash TP, Bhat B. 2′-Modified oligonucleotides for antisense therapeutics. Curr Top Med Chem. 2007;7:641–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

