Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases
- PMID: 21756134
- PMCID: PMC3406932
- DOI: 10.1146/annurev.nutr.012809.104635
Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases
Abstract
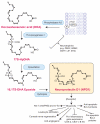
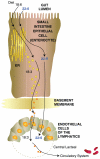
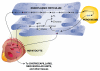
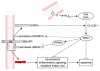
Essential polyunsaturated fatty acids (PUFAs) are critical nutritional lipids that must be obtained from the diet to sustain homeostasis. Omega-3 and -6 PUFAs are key components of biomembranes and play important roles in cell integrity, development, maintenance, and function. The essential omega-3 fatty acid family member docosahexaenoic acid (DHA) is avidly retained and uniquely concentrated in the nervous system, particularly in photoreceptors and synaptic membranes. DHA plays a key role in vision, neuroprotection, successful aging, memory, and other functions. In addition, DHA displays anti-inflammatory and inflammatory resolving properties in contrast to the proinflammatory actions of several members of the omega-6 PUFAs family. This review discusses DHA signalolipidomics, comprising the cellular/tissue organization of DHA uptake, its distribution among cellular compartments, the organization and function of membrane domains rich in DHA-containing phospholipids, and the cellular and molecular events revealed by the uncovering of signaling pathways regulated by DHA and docosanoids, the DHA-derived bioactive lipids, which include neuroprotectin D1 (NPD1), a novel DHA-derived stereoselective mediator. NPD1 synthesis agonists include neurotrophins and oxidative stress; NPD1 elicits potent anti-inflammatory actions and prohomeostatic bioactivity, is anti-angiogenic, promotes corneal nerve regeneration, and induces cell survival. In the context of DHA signalolipidomics, this review highlights aging and the evolving studies on the significance of DHA in Alzheimer's disease, macular degeneration, Parkinson's disease, and other brain disorders. DHA signalolipidomics in the nervous system offers emerging targets for pharmaceutical intervention and clinical translation.
Figures



References
-
- Agostoni C. Role of long-chain polyunsaturated fatty acids in the first year of life. J. Pediatr. Gastroenterol. Nutr. 2008;47:S41–44. - PubMed
-
- Anders A, Gilbert S, Garten W, Postina R, Fahrenholz F. Regulation of the alpha-secretase ADAM10 by its prodomain and proprotein convertases. FASEB J. 2001;15:1837–39. - PubMed
-
- Anderson RE, Maude MB, McClellan M, Matthes MT, Yasumura D, LaVail MM. Low docosahexaenoic acid levels in rod outer segments of rats with P23H and S334ter rhodopsin mutations. Mol. Vis. 2002;8:351–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

