tPA contributes to impaired NMDA cerebrovasodilation after traumatic brain injury through activation of JNK MAPK
- PMID: 21756552
- PMCID: PMC3523283
- DOI: 10.1179/016164110X12807570509853
tPA contributes to impaired NMDA cerebrovasodilation after traumatic brain injury through activation of JNK MAPK
Abstract
Objective: N-methyl-D-aspartate (NMDA)-induced pial artery dilation (PAD) is reversed to vasoconstriction after fluid percussion brain injury (FPI). Tissue type plasminogen activator (tPA) is up-regulated and the tPA antagonist, EEIIMD, prevents impaired NMDA PAD after FPI. Mitogen-activated protein kinase (MAPK), a family of at least three kinases, ERK, p38, and JNK, is also up-regulated after traumatic brain injury (TBI). We hypothesize that tPA impairs NMDA-induced cerebrovasodilation after FPI in a MAPK isoform-dependent mechanism.
Methods: Lateral FPI was induced in newborn pigs. The closed cranial window technique was used to measure pial artery diameter and to collect cerebrospinal fluid (CSF). ERK, p38, and JNK MAPK concentrations in CSF were quantified by ELISA.
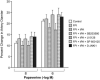
Results: CSF JNK MAPK was increased by FPI, increased further by tPA, but blocked by JNK antagonists SP600125 and D-JNKI1. FPI modestly increased p38 and ERK isoforms of MAPK. NMDA-induced PAD was reversed to vasoconstriction after FPI, whereas dilator responses to papaverine were unchanged. tPA, in post-FPI CSF concentration, potentiated NMDA-induced vasoconstriction while papaverine dilation was unchanged. SP 600125 and D-JNKI1, blocked NMDA-induced vasoconstriction and fully restored PAD. The ERK antagonist U 0126 partially restored NMDA-induced PAD, while the p38 inhibitor SB203580 aggravated NMDA-induced vasoconstriction observed in the presence of tPA after FPI.
Discussion: These data indicate that tPA contributes to impairment of NMDA-mediated cerebrovasodilation after FPI through JNK, while p38 may be protective. These data suggest that inhibition of the endogenous plasminogen activator system and JNK may improve cerebral hemodynamic outcome post-TBI.
Figures
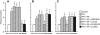
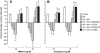
References
-
- Rodriguez JG. Childhood injuries in the United States. A priority issue. Am J. Dis Child. 1990;144:625–626. - PubMed
-
- Wei EP, Dietrich WD, Povlishock JT, Navari RM, Kontos HA. Functional, morphological, and metabolic abnormalities of the cerebral microcirculation after concussive brain injury in cats. Circ Res. 1980;46:37–47. - PubMed
-
- Richards HK, Simac S, Piechnik S, Pickard JD. Uncoupling of cerebral blood flow and metabolism after cerebral contusion in the rat. J Cereb Blood Flow Metab. 2001;21:779–781. - PubMed
-
- Bruce DA, Alavi A, Bilaniuk L, Dolinskas C, Obrist W, Uzzell B. Diffuse cerebral swelling following head injuries in children; the syndrome of malignant brain edema. J Neurosurg. 1981;54:170–178. - PubMed
-
- Adelson PD, Clyde B, Kochanek PM, Wisniewski SR, Marion DW, Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr Neurosurg. 1997;26:200–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA083121/CA/NCI NIH HHS/United States
- HL76206/HL/NHLBI NIH HHS/United States
- HD57355/HD/NICHD NIH HHS/United States
- P01 HL076406/HL/NHLBI NIH HHS/United States
- R01 HL076206/HL/NHLBI NIH HHS/United States
- NS53410/NS/NINDS NIH HHS/United States
- CA83121/CA/NCI NIH HHS/United States
- HL81864/HL/NHLBI NIH HHS/United States
- T32 HL007971/HL/NHLBI NIH HHS/United States
- R01 HD057355/HD/NICHD NIH HHS/United States
- HL82545/HL/NHLBI NIH HHS/United States
- R01 NS053410/NS/NINDS NIH HHS/United States
- R01 HL077760/HL/NHLBI NIH HHS/United States
- HL76406/HL/NHLBI NIH HHS/United States
- HL77760/HL/NHLBI NIH HHS/United States
- R21 HL081864/HL/NHLBI NIH HHS/United States
- HL07971/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
