Immunohistochemical analysis of TIMP-2 and collagen types I and IV in experimental spinal cord ischemia-reperfusion injury in rats
- PMID: 21756563
- PMCID: PMC3127370
- DOI: 10.1179/107902611X12972448729648
Immunohistochemical analysis of TIMP-2 and collagen types I and IV in experimental spinal cord ischemia-reperfusion injury in rats
Abstract
Background: Thoracic and thoracoabdominal aortic intervention carries a significant risk of spinal cord ischemia. The pathophysiologic mechanisms that cause hypoxic/ischemic injury to the spinal cord have not been totally explained. In normal spinal cord, neurons and glial cells do not express type IV collagen. Type IV collagen produced by reactive astrocytes is reported to participate in glial scar formation. Tissue inhibitors of metalloproteinases (TIMPs) are endogenous inhibitors that regulate the activity of the matrix metalloproteinases (MMPs). TIMP-2 binds strongly with MMP-2, facilitating activation by membrane-type MMP. Imbalance between TIMPs and MMPs can lead to excessive degradation of matrix components. Type IV collagen involved in the blood-brain barrier disruption and glial scar formation, TIMP-2 influences MMP-2 that controls degradation of collagen I and IV.
Objective: To examine the immunohistochemical analysis of TIMP-2 and collagen types I-IV in experimental spinal cord ischemia-reperfusion in rats.
Methods: Thirty-two male Wistar rats weighing 250-300 g were divided into four groups: group S: sham group (n = 8); group 0P: 30-minute occlusion without perfusion (n = 8); group 3P: 30-minute occlusion and 3-hour perfusion (n = 8); and group 24P: 30-minute occlusion and 24-hour perfusion (n = 8). Infrarenal aorta was cross-clamped at two sites by using two aneurysm clips for 30 minutes. Reperfusion was provided after removal of the clips. Lumbar spinal cord segments were removed for immunohistochemical analysis.
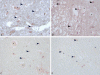
Results: TIMP-2 and collagen staining in 3-hour perfused (3P) group were nearly the same with sham group (S). TIMP-2 and collagen staining increased in the 24-hour perfused group.
Conclusion: Alterations in collagen levels may relate to the biphasic breakdown of the blood-brain barrier and collagen staining in new cell types with relation to glial scar formation. Our results demonstrate that 3-hour perfusion after occlusion in hypoxic/ischemic spinal cord injury seems to be the critical reversible period.
Figures


References
-
- Erten SF, Kocak A, Ozdemir I, Aydemir S, Colak A, Reeder BS. Protective effect of melatonin on experimental spinal cordischemia. Spinal Cord 2003;4(10):533–38 - PubMed
-
- Emmez H, Yildirim Z, Kale A, Tönge M, Durdağ E, Börcek AO, et al. Anti-apoptotic and neuroprotective effects of alpha-lipoic acid on spinal cord ischemia-reperfusion injury in rabbits. Acta Neurochir (Wien) 2010;152(9):1591–600 - PubMed
-
- Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma 2006;23(3–4):318–34 - PubMed
-
- Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 2001;24(5):254–64 - PubMed
-
- Dumont RJ, Verma S, Okonkwo DO, Hurlbert RJ, Boulos PT, Ellegala DB, et al. Acute spinal cord injury, part II: contemporary pharmacotherapy. Clin Neuropharmacol 2001;24(5):265–79 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
