The cytochrome bd respiratory oxygen reductases
- PMID: 21756872
- PMCID: PMC3171616
- DOI: 10.1016/j.bbabio.2011.06.016
The cytochrome bd respiratory oxygen reductases
Abstract
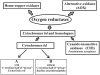
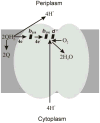
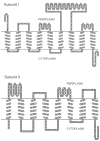
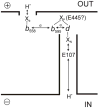
Cytochrome bd is a respiratory quinol: O₂ oxidoreductase found in many prokaryotes, including a number of pathogens. The main bioenergetic function of the enzyme is the production of a proton motive force by the vectorial charge transfer of protons. The sequences of cytochromes bd are not homologous to those of the other respiratory oxygen reductases, i.e., the heme-copper oxygen reductases or alternative oxidases (AOX). Generally, cytochromes bd are noteworthy for their high affinity for O₂ and resistance to inhibition by cyanide. In E. coli, for example, cytochrome bd (specifically, cytochrome bd-I) is expressed under O₂-limited conditions. Among the members of the bd-family are the so-called cyanide-insensitive quinol oxidases (CIO) which often have a low content of the eponymous heme d but, instead, have heme b in place of heme d in at least a majority of the enzyme population. However, at this point, no sequence motif has been identified to distinguish cytochrome bd (with a stoichiometric complement of heme d) from an enzyme designated as CIO. Members of the bd-family can be subdivided into those which contain either a long or a short hydrophilic connection between transmembrane helices 6 and 7 in subunit I, designated as the Q-loop. However, it is not clear whether there is a functional consequence of this difference. This review summarizes current knowledge on the physiological functions, genetics, structural and catalytic properties of cytochromes bd. Included in this review are descriptions of the intermediates of the catalytic cycle, the proposed site for the reduction of O₂, evidence for a proton channel connecting this active site to the bacterial cytoplasm, and the molecular mechanism by which a membrane potential is generated.
2011 Elsevier B.V. All rights reserved.
Figures


References
-
- Brunori M, Giuffre A, Sarti P. Cytochrome c oxidase, ligands and electrons. J Inorg Biochem. 2005;99:324–336. - PubMed
-
- Branden G, Gennis RB, Brzezinski P. Transmembrane proton translocation by cytochrome c oxidase. Biochim Biophys Acta. 2006;1757:1052–1063. - PubMed
-
- Wikstrom M, Verkhovsky MI. Mechanism and energetics of proton translocation by the respiratory heme-copper oxidases. Biochim Biophys Acta. 2007;1767:1200–1214. - PubMed
-
- Belevich I, Verkhovsky MI. Molecular mechanism of proton translocation by cytochrome c oxidase. Antioxid Redox Signal. 2008;10:1–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

