A novel method for producing mono-biotinylated, biologically active neurotrophic factors: an essential reagent for single molecule study of axonal transport
- PMID: 21756937
- PMCID: PMC3158612
- DOI: 10.1016/j.jneumeth.2011.06.020
A novel method for producing mono-biotinylated, biologically active neurotrophic factors: an essential reagent for single molecule study of axonal transport
Abstract
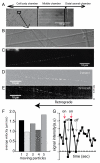
In this report, we describe a novel method for producing mature and biologically active mono-biotinylated nerve growth factors (mBtNGF) that can be used for single molecule studies of real-time movement of neurotrophins within axons of neurons. We inserted an AviTag sequence into the C-terminal of the full length mouse preproNGF cDNA and cloned the fusion construct into a pcDNA3.1 mammalian expression vector. We also subcloned the Escherichia coli biotin ligase, BirA, into a pcDNA3.1 vector. These two plasmids were then transiently co-expressed in HEK293FT cells. As a result, the AviTag located in the C-terminal of preproNGF was selectively ligated to a single biotin by BirA. The prepro sequence of NGF was subsequently cleaved within the cell. Mature mono-biotinylated NGF (mBtNGF) was secreted into cell culture media and was purified using Ni resin. We carried out activity assays and our results showed that mBtNGF retained biological activities that were comparable to normal NGF purified from mouse sub maxillary glands. We further verified the biotinylation efficiency of mBtNGF and the level of non-biotinylated NGF was virtually undetectable in the final preparation. Finally, by conjugating to quantum-dot streptavidin, mBtNGF was successfully used for single molecule study of axonal NGF trafficking in neurons.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

