Human CRB1-associated retinal degeneration: comparison with the rd8 Crb1-mutant mouse model
- PMID: 21757580
- PMCID: PMC3176016
- DOI: 10.1167/iovs.11-7701
Human CRB1-associated retinal degeneration: comparison with the rd8 Crb1-mutant mouse model
Abstract
Purpose: To investigate the human disease due to CRB1 mutations and compare results with the Crb1-mutant rd8 mouse.
Methods: Twenty-two patients with CRB1 mutations were studied. Function was assessed with perimetry and electroretinography (ERG) and retinal structure with optical coherence tomography (OCT). Cortical structure and function were quantified with magnetic resonance imaging (MRI). Rd8 mice underwent ERG, OCT, and retinal histopathology.
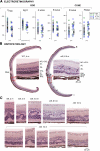
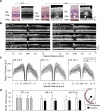
Results: Visual acuities ranged from 20/25 to light perception. Rod ERGs were not detectable; small cone signals were recordable. By perimetry, small central visual islands were separated by midperipheral scotomas from far temporal peripheral islands. The central islands were cone mediated, whereas the peripheral islands retained some rod function. With OCT, there were small foveal islands of thinned outer nuclear layer (ONL) surrounded by thick delaminated retina with intraretinal hyperreflective lesions. MRI showed structurally normal optic nerves and only subtle changes to occipital lobe white and gray matter. Functional MRI indicated that whole-brain responses from patients were of reduced amplitude and spatial extent compared with those of normal controls. Rd8 mice had essentially normal ERGs; OCT and histopathology showed patchy retinal disorganization with pseudorosettes more pronounced in ventral than in dorsal retina. Photoreceptor degeneration was associated with dysplastic regions.
Conclusions: CRB1 mutations lead to early-onset severe loss of vision with thickened, disorganized, nonseeing retina. Impaired peripheral vision can persist in late disease stages. Rd8 mice also have a disorganized retina, but there is sufficient photoreceptor integrity to produce largely normal retinal function. Differences between human and mouse diseases will complicate proof-of-concept studies intended to advance treatment initiatives.
Figures



References
-
- Bazellieres E, Assemat E, Arsanto JP, Le Bivic A, Massey-Harroche D. Crumbs proteins in epithelial morphogenesis. Front Biosci. 2009;14:2149–2169 - PubMed
-
- den Hollander AI, ten Brink JB, de Kok YJ, et al. Mutations in a human homologue of drosophila crumbs cause retinitis pigmentosa (RP12). Nat Genet. 1999;23:217–221 - PubMed
-
- Lotery AJ, Jacobson SG, Fishman GA, et al. Mutations in the CRB1 gene cause leber congenital amaurosis. Arch Ophthalmol. 2001;119:415–420 - PubMed
-
- Jacobson SG, Cideciyan AV, Aleman TS, et al. Crumbs homolog 1 (CRB1) mutations result in a thick human retina with abnormal lamination. Hum Mol Genet. 2003;12:1073–1078 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

