Calpain, not caspase, is the causative protease for hypoxic damage in cultured monkey retinal cells
- PMID: 21757584
- PMCID: PMC3207712
- DOI: 10.1167/iovs.11-7497
Calpain, not caspase, is the causative protease for hypoxic damage in cultured monkey retinal cells
Abstract
Purpose: Cell death occurring in human retina during AMD, high IOP, and diabetic retinopathy could be caused by activation of calpain or caspase proteolytic enzymes. The purpose of the present study was to determine whether calpains and/or caspase-3 were involved in cell death during retinal hypoxia in a monkey model.
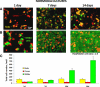
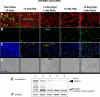
Methods: Dissociated monkey retinal cells were cultured for two weeks and subjected to 24-hour hypoxia/24-hour reoxygenation. TUNEL staining and immunostaining for Müller and photoreceptor markers were used to detect which retinal cell types were damaged.
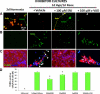
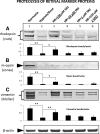
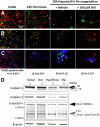
Results: Culturing dissociated monkey retina cells for two weeks resulted in proliferation of Müller cells and maintenance of some rod and cone photoreceptor cells, as identified by vimentin, recoverin, and rhodopsin immunocytochemical staining. Hypoxia/reoxygenation increased the number of cells staining positive for TUNEL. Immunoblotting showed that the calpain-specific 145 kDa α-spectrin breakdown product (SBDP) increased in hypoxic cells, but no caspase-specific 120 kDa α-spectrin breakdown product was detected. TUNEL staining and proteolysis were significantly reduced in the retinal cells treated with 10 and 100 μM calpain inhibitor SNJ-1945. Caspase inhibitor, z-VAD, did not inhibit cell damage from hypoxia/reoxygenation. Intact pro-caspase-3 was in fact cleaved by activated calpain during hypoxia/reoxygenation to pre 29 kDa caspase-3 and 24 kDa inactive fragments. No 17 and 12 kDa fragments, which form the active caspase-3 hetero-dimer, were detected. Calpain-induced cleavage of caspase was inhibited by SNJ-1945.
Conclusions: Calpain, not caspase-3, was involved in hypoxic damage in cultured monkey retinal cells.
Figures


Similar articles
-
Hypoxia Activates Calpains in the Nerve Fiber Layer of Monkey Retinal Explants.Invest Ophthalmol Vis Sci. 2015 Sep;56(10):6049-57. doi: 10.1167/iovs.15-17360. Invest Ophthalmol Vis Sci. 2015. PMID: 26393472 Free PMC article.
-
Activation of Cytosolic Calpain, Not Caspase, Is Underlying Mechanism for Hypoxic RGC Damage in Human Retinal Explants.Invest Ophthalmol Vis Sci. 2020 Nov 2;61(13):13. doi: 10.1167/iovs.61.13.13. Invest Ophthalmol Vis Sci. 2020. PMID: 33156340 Free PMC article.
-
Calcium-dependent pathway as a primary cause of hypoxic RGC damage in monkey retinal explants.PLoS One. 2025 Jul 11;20(7):e0327246. doi: 10.1371/journal.pone.0327246. eCollection 2025. PLoS One. 2025. PMID: 40644465 Free PMC article.
-
The pathogenic activation of calpain: a marker and mediator of cellular toxicity and disease states.Int J Exp Pathol. 2000 Oct;81(5):323-39. doi: 10.1111/j.1365-2613.2000.00169.x. Int J Exp Pathol. 2000. PMID: 11168679 Free PMC article. Review.
-
The role of calcium-activated protease calpain in experimental retinal pathology.Surv Ophthalmol. 2008 Mar-Apr;53(2):150-63. doi: 10.1016/j.survophthal.2007.12.006. Surv Ophthalmol. 2008. PMID: 18348880 Free PMC article. Review.
Cited by
-
Activation of the mitochondrial caspase pathway and subsequent calpain activation in monkey RPE cells cultured under zinc depletion.Eye (Lond). 2014 Jan;28(1):85-92. doi: 10.1038/eye.2013.239. Epub 2013 Nov 8. Eye (Lond). 2014. PMID: 24202052 Free PMC article.
-
Hypoxia Activates Calpains in the Nerve Fiber Layer of Monkey Retinal Explants.Invest Ophthalmol Vis Sci. 2015 Sep;56(10):6049-57. doi: 10.1167/iovs.15-17360. Invest Ophthalmol Vis Sci. 2015. PMID: 26393472 Free PMC article.
-
Calpain-specific breakdown fragment in human drusen.Histol Histopathol. 2024 Feb;39(2):165-175. doi: 10.14670/HH-18-635. Epub 2023 Jun 1. Histol Histopathol. 2024. PMID: 37314158
-
Neurodegeneration in the pathogenesis of diabetic retinopathy: molecular mechanisms and therapeutic implications.Curr Med Chem. 2013;20(26):3241-50. doi: 10.2174/09298673113209990027. Curr Med Chem. 2013. PMID: 23745549 Free PMC article. Review.
-
Proteomic Analysis Revealed the Important Role of Vimentin in Human Cervical Carcinoma HeLa Cells Treated With Gambogic Acid.Mol Cell Proteomics. 2016 Jan;15(1):26-44. doi: 10.1074/mcp.M115.053272. Epub 2015 Oct 23. Mol Cell Proteomics. 2016. PMID: 26499837 Free PMC article.
References
-
- Kawasaki A, Otori Y, Barnstable CJ. Muller cell protection of rat retinal ganglion cells from glutamate and nitric oxide neurotoxicity. Invest Ophthalmol Vis Sci. 2000;41:3444–3450 - PubMed
-
- Bringmann A, Pannicke T, Grosche J, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424 - PubMed
-
- Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302 - PubMed
-
- Lawrence JM, Singhal S, Bhatia B, et al. MIO-M1 cells and similar Muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007:25;2033–2043 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

