NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions
- PMID: 21757615
- PMCID: PMC3172795
- DOI: 10.1182/blood-2011-04-347070
NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions
Abstract
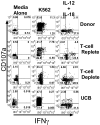
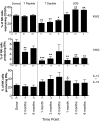
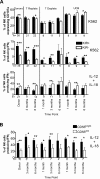
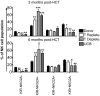
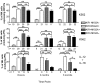
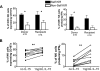
Natural killer (NK) cells mediate GVL effects after allogeneic hematopoietic cell transplantation (allo-HCT) by the production of inflammatory cytokines and by direct target lysis. The acquisition of both functions was presumed to be developmentally linked, but this linkage remained unstudied after allo-HCT. We tested the cytokine production and degranulation of reconstituting NK cells after adult unrelated donor or umbilical cord blood grafting. Recipients of T cell-depleted transplants, receiving no immune suppression, showed diminished NK cell degranulation. In contrast, degranulation was normal or increased after T-cell replete transplants given with immune suppression. Strikingly, target cell-induced IFNγ production was markedly diminished in all transplant settings, especially with T cell-depleted or naive T cell-containing umbilical cord blood grafts, suggesting a role for T cells in NK education. Although degranulation was similar in the KIR(+) and KIR(-) populations that coexpressed NKG2A, target cell-induced IFNγ production was limited to the subset of NK cells expressing KIR inhibited by self-ligands. Thus, cytokine production and cytotoxic function do not consistently coexist in NK cells reconstituting after allo-HCT. Exposure to IL-15 rapidly increased target-inducible IFNγ production, indicative of IL-15's potential as a therapeutic tool to enhance NK cell function to protect against infection and relapse after allo-HCT.
Figures

References
-
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. - PubMed
-
- Miller JS, Warren EH, van den Brink MR, Ritz J, Shlomchik WD, Murphy WJ, et al. NCI First International Workshop on The Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT: graft-versus-tumor/leukemia reaction. Biol Blood Marrow Transplant. 2010;16(5):565–586. - PMC - PubMed
-
- Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306(5701):1517–1519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

