S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation
- PMID: 21757621
- PMCID: PMC3175787
- DOI: 10.1182/blood-2011-02-331579
S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation
Abstract
Platelet activation and thrombus formation are under the control of signaling systems that integrate cellular homeostasis with cytoskeletal dynamics. Here, we identify a role for the ribosome protein S6 kinase (S6K1) and its upstream regulator mTOR in the control of platelet activation and aggregate formation under shear flow. Platelet engagement of fibrinogen initiated a signaling cascade that triggered the activation of S6K1 and Rac1. Fibrinogen-induced S6K1 activation was abolished by inhibitors of Src kinases, but not Rac1 inhibitors, demonstrating that S6K1 acts upstream of Rac1. S6K1 and Rac1 interacted in a protein complex with the Rac1 GEF TIAM1 and colocalized with actin at the platelet lamellipodial edge, suggesting that S6K1 and Rac1 work together to drive platelet spreading. Pharmacologic inhibitors of mTOR and S6K1 blocked Rac1 activation and prevented platelet spreading on fibrinogen, but had no effect on Src or FAK kinase activation. mTOR inhibitors dramatically reduced collagen-induced platelet aggregation and promoted the destabilization of platelet aggregates formed under shear flow conditions. Together, these results reveal novel roles for S6K1 and mTOR in the regulation of Rac1 activity and provide insights into the relationship between the pharmacology of the mTOR system and the molecular mechanisms of platelet activation.
Figures
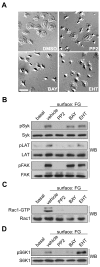
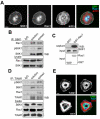

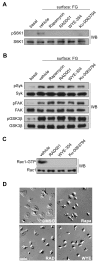


Comment in
-
Regulation of the mTOR-Rac1 axis in platelet function.Small GTPases. 2012 Jan-Mar;3(1):67-70. doi: 10.4161/sgtp.19137. Small GTPases. 2012. PMID: 22714420 Free PMC article.
References
-
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227–1234. - PubMed
-
- Watson SP. Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Curr Pharm Des. 2009;15(12):1358–1372. - PubMed
-
- Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3(8):1752–1762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

