Telomere length is a determinant of emphysema susceptibility
- PMID: 21757622
- PMCID: PMC3208661
- DOI: 10.1164/rccm.201103-0520OC
Telomere length is a determinant of emphysema susceptibility
Abstract
Rationale: Germline mutations in the enzyme telomerase cause telomere shortening, and have their most common clinical manifestation in age-related lung disease that manifests as idiopathic pulmonary fibrosis. Short telomeres are also a unique heritable trait that is acquired with age.
Objectives: We sought to understand the mechanisms by which telomerase deficiency contributes to lung disease.
Methods: We studied telomerase null mice with short telomeres.
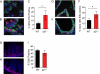
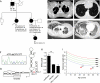
Measurements and main results: Although they have no baseline histologic defects, when mice with short telomeres are exposed to chronic cigarette smoke, in contrast with controls, they develop emphysematous air space enlargement. The emphysema susceptibility did not depend on circulating cell genotype, because mice with short telomeres developed emphysema even when transplanted with wild-type bone marrow. In lung epithelium, cigarette smoke exposure caused additive DNA damage to telomere dysfunction, which limited their proliferative recovery, and coincided with a failure to down-regulate p21, a mediator of cellular senescence, and we show here, a determinant of alveolar epithelial cell cycle progression. We also report early onset of emphysema, in addition to pulmonary fibrosis, in a family with a germline deletion in the Box H domain of the RNA component of telomerase.
Conclusions: Our data indicate that short telomeres lower the threshold of cigarette smoke-induced damage, and implicate telomere length as a genetic susceptibility factor in emphysema, potentially contributing to its age-related onset in humans.
Figures
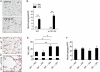


Comment in
-
Merging personalized medicine and biology of aging in chronic obstructive pulmonary disease.Am J Respir Crit Care Med. 2011 Oct 15;184(8):864-6. doi: 10.1164/rccm.201108-1486ED. Am J Respir Crit Care Med. 2011. PMID: 22003144 No abstract available.
-
Is telomeropathy the explanation for combined pulmonary fibrosis and emphysema syndrome?: report of a family with TERT mutation.Am J Respir Crit Care Med. 2014 Mar 15;189(6):753-4. doi: 10.1164/rccm.201309-1724LE. Am J Respir Crit Care Med. 2014. PMID: 24628319 No abstract available.
-
Reply: telomerase makes connections between pulmonary fibrosis and emphysema.Am J Respir Crit Care Med. 2014 Mar 15;189(6):754-5. doi: 10.1164/rccm.201401-0019LE. Am J Respir Crit Care Med. 2014. PMID: 24628320 Free PMC article. No abstract available.
References
-
- Fowler RW. Ageing and lung function. Age Ageing 1985;14:209–215 - PubMed
-
- Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, Israel-Biet D, Court-Fortune I, Valeyre D, Cordier JF. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J 2005;26:586–593 - PubMed
-
- Mason RJ, Buist AS, Fisher EB, Merchant JA, Samet JM, Welsh CH. Cigarette smoking and health. Am Rev Respir Dis 1985;132:1133–1136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

