Asymmetric cancer cell division regulated by AKT
- PMID: 21757645
- PMCID: PMC3150943
- DOI: 10.1073/pnas.1109632108
Asymmetric cancer cell division regulated by AKT
Abstract
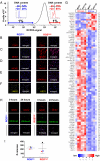
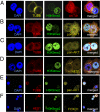
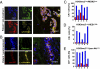
Human tumors often contain slowly proliferating cancer cells that resist treatment, but we do not know precisely how these cells arise. We show that rapidly proliferating cancer cells can divide asymmetrically to produce slowly proliferating "G0-like" progeny that are enriched following chemotherapy in breast cancer patients. Asymmetric cancer cell division results from asymmetric suppression of AKT/PKB kinase signaling in one daughter cell during telophase of mitosis. Moreover, inhibition of AKT signaling with small-molecule drugs can induce asymmetric cancer cell division and the production of slow proliferators. Cancer cells therefore appear to continuously flux between symmetric and asymmetric division depending on the precise state of their AKT signaling network. This model may have significant implications for understanding how tumors grow, evade treatment, and recur.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

