Histidine-rich glycoprotein binds fibrin(ogen) with high affinity and competes with thrombin for binding to the gamma'-chain
- PMID: 21757718
- PMCID: PMC3162390
- DOI: 10.1074/jbc.M111.253831
Histidine-rich glycoprotein binds fibrin(ogen) with high affinity and competes with thrombin for binding to the gamma'-chain
Abstract
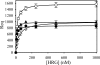
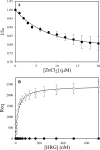
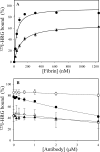
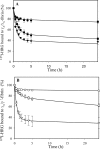
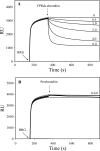
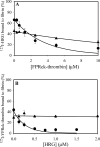
Histidine-rich glycoprotein (HRG) is an abundant protein that binds fibrinogen and other plasma proteins in a Zn(2+)-dependent fashion but whose function is unclear. HRG has antimicrobial activity, and its incorporation into fibrin clots facilitates bacterial entrapment and killing and promotes inflammation. Although these findings suggest that HRG contributes to innate immunity and inflammation, little is known about the HRG-fibrin(ogen) interaction. By immunoassay, HRG-fibrinogen complexes were detected in Zn(2+)-supplemented human plasma, a finding consistent with a high affinity interaction. Surface plasmon resonance determinations support this concept and show that in the presence of Zn(2+), HRG binds the predominant γ(A)/γ(A)-fibrinogen and the γ-chain elongated isoform, γ(A)/γ'-fibrinogen, with K(d) values of 9 nm. Likewise, (125)I-labeled HRG binds γ(A)/γ(A)- or γ(A)/γ'-fibrin clots with similar K(d) values when Zn(2+) is present. There are multiple HRG binding sites on fibrin(ogen) because HRG binds immobilized fibrinogen fragment D or E and γ'-peptide, an analog of the COOH terminus of the γ'-chain that mediates the high affinity interaction of thrombin with γ(A)/γ'-fibrin. Thrombin competes with HRG for γ'-peptide binding and displaces (125)I-HRG from γ(A)/γ'-fibrin clots and vice versa. Taken together, these data suggest that (a) HRG circulates in complex with fibrinogen and that the complex persists upon fibrin formation, and (b) by competing with thrombin for γ(A)/γ'-fibrin binding, HRG may modulate coagulation. Therefore, the HRG-fibrin interaction may provide a novel link between coagulation, innate immunity, and inflammation.
Figures
Similar articles
-
Evidence that both exosites on thrombin participate in its high affinity interaction with fibrin.J Biol Chem. 2003 Jun 13;278(24):21584-91. doi: 10.1074/jbc.M300545200. Epub 2003 Apr 7. J Biol Chem. 2003. PMID: 12682049
-
Evidence that catalytically-inactivated thrombin forms non-covalently linked dimers that bridge between fibrin/fibrinogen fibers and enhance fibrin polymerization.Biophys Chem. 2004 Jul 1;110(1-2):93-100. doi: 10.1016/j.bpc.2004.01.007. Biophys Chem. 2004. PMID: 15223147
-
Long range communication between exosites 1 and 2 modulates thrombin function.J Biol Chem. 2009 Sep 18;284(38):25620-9. doi: 10.1074/jbc.M109.000042. Epub 2009 Jul 9. J Biol Chem. 2009. PMID: 19589779 Free PMC article.
-
Antithrombin I. Inhibition of thrombin generation in plasma by fibrin formation.Thromb Haemost. 2003 Jan;89(1):9-12. Thromb Haemost. 2003. PMID: 12540947 Review.
-
The structure and biological features of fibrinogen and fibrin.Ann N Y Acad Sci. 2001;936:11-30. doi: 10.1111/j.1749-6632.2001.tb03491.x. Ann N Y Acad Sci. 2001. PMID: 11460466 Review.
Cited by
-
Differences in plasma fibrin clot composition in patients with thrombotic antiphospholipid syndrome compared with venous thromboembolism.Sci Rep. 2018 Nov 23;8(1):17301. doi: 10.1038/s41598-018-35034-x. Sci Rep. 2018. PMID: 30470809 Free PMC article.
-
Fibrinolysis Shutdown in COVID-19: Clinical Manifestations, Molecular Mechanisms, and Therapeutic Implications.J Am Coll Surg. 2021 Jun;232(6):995-1003. doi: 10.1016/j.jamcollsurg.2021.02.019. Epub 2021 Mar 22. J Am Coll Surg. 2021. PMID: 33766727 Free PMC article. Review.
-
Plasma protein profiling of Mild Cognitive Impairment and Alzheimer's disease using iTRAQ quantitative proteomics.Proteome Sci. 2014 Jan 17;12(1):5. doi: 10.1186/1477-5956-12-5. Proteome Sci. 2014. PMID: 24433274 Free PMC article.
-
The Interplay between Non-Esterified Fatty Acids and Plasma Zinc and Its Influence on Thrombotic Risk in Obesity and Type 2 Diabetes.Int J Mol Sci. 2021 Sep 20;22(18):10140. doi: 10.3390/ijms221810140. Int J Mol Sci. 2021. PMID: 34576303 Free PMC article. Review.
-
A cross-sectional analysis of zinc and copper levels and their relationship to cardiovascular disease risk markers in Qatar biobank participants.Front Cardiovasc Med. 2024 Jan 5;10:1305588. doi: 10.3389/fcvm.2023.1305588. eCollection 2023. Front Cardiovasc Med. 2024. PMID: 38250034 Free PMC article.
References
-
- Henschen A., Lottspeich F., Kehl M., Southan C. (1983) Ann. N.Y. Acad. Sci. 408, 28–43 - PubMed
-
- Wolfenstein-Todel C., Mosesson M. W. (1981) Biochemistry 20, 6146–6149 - PubMed
-
- Chung D. W., Davie E. W. (1984) Biochemistry 23, 4232–4236 - PubMed
-
- Fornace A. J., Jr., Cummings D. E., Comeau C. M., Kant J. A., Crabtree G. R. (1984) J. Biol. Chem. 259, 12826–12830 - PubMed
-
- Meh D. A., Siebenlist K. R., Mosesson M. W. (1996) J. Biol. Chem. 271, 23121–23125 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

