mSIN1 protein mediates SGK1 protein interaction with mTORC2 protein complex and is required for selective activation of the epithelial sodium channel
- PMID: 21757730
- PMCID: PMC3162425
- DOI: 10.1074/jbc.M111.257592
mSIN1 protein mediates SGK1 protein interaction with mTORC2 protein complex and is required for selective activation of the epithelial sodium channel
Abstract
The mammalian target of rapamycin (mTOR) plays a central role in the regulation of a number of cellular processes including growth, metabolism, and ion transport. mTOR is found in two multiprotein complexes, mTORC1 and mTORC2, which phosphorylate distinct substrates and regulate distinct cellular processes. SGK1 is an mTORC2 substrate, which is a key regulator of epithelial Na(+) transport mediated by the epithelial sodium channel. Although it is known that SGK1 physically interacts with mTORC2, it is unknown which mTORC2 component mediates this interaction or whether this interaction plays a physiologically relevant role in specific activation of SGK1. Here we identify mSIN1 as the mTORC2 component that mediates interaction with SGK1 and demonstrate that this interaction is required for SGK1 phosphorylation and epithelial sodium channel activation. We used the yeast two-hybrid system coupled with random mutagenesis to identify a mutant mSIN1 (mSIN1/Q68H), which does not interact with SGK1. Expression of this mutant does not restore SGK1 phosphorylation to wild-type levels in mSIN1-deficient murine embryo fibroblasts. Furthermore, in kidney epithelial cells, mSIN1/Q68H has a dominant-negative effect on SGK1 phosphorylation and on SGK1-dependent Na(+) transport. Interestingly, this interaction appears to be specific in that another mTORC2 substrate, Akt, does not interact with mSIN1, and its phosphorylation and activity are unaffected by the Q68H mutation. These data support the conclusion that mTORC2 uses distinct strategies to phosphorylate different substrates and suggest a mechanism for mTORC2 specificity in the regulation of diverse cellular processes.
Figures



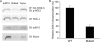
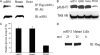
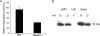
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

