Linkage of organic anion transporter-1 to metabolic pathways through integrated "omics"-driven network and functional analysis
- PMID: 21757732
- PMCID: PMC3173137
- DOI: 10.1074/jbc.M111.272534
Linkage of organic anion transporter-1 to metabolic pathways through integrated "omics"-driven network and functional analysis
Abstract
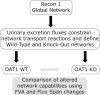
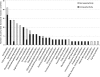
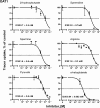
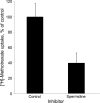
The main kidney transporter of many commonly prescribed drugs (e.g. penicillins, diuretics, antivirals, methotrexate, and non-steroidal anti-inflammatory drugs) is organic anion transporter-1 (OAT1), originally identified as NKT (Lopez-Nieto, C. E., You, G., Bush, K. T., Barros, E. J., Beier, D. R., and Nigam, S. K. (1997) J. Biol. Chem. 272, 6471-6478). Targeted metabolomics in knockouts have shown that OAT1 mediates the secretion or reabsorption of many important metabolites, including intermediates in carbohydrate, fatty acid, and amino acid metabolism. This observation raises the possibility that OAT1 helps regulate broader metabolic activities. We therefore examined the potential roles of OAT1 in metabolic pathways using Recon 1, a functionally tested genome-scale reconstruction of human metabolism. A computational approach was used to analyze in vivo metabolomic as well as transcriptomic data from wild-type and OAT1 knock-out animals, resulting in the implication of several metabolic pathways, including the citric acid cycle, polyamine, and fatty acid metabolism. Validation by in vitro and ex vivo analysis using Xenopus oocyte, cell culture, and kidney tissue assays demonstrated interactions between OAT1 and key intermediates in these metabolic pathways, including previously unknown substrates, such as polyamines (e.g. spermine and spermidine). A genome-scale metabolic network reconstruction generated some experimentally supported predictions for metabolic pathways linked to OAT1-related transport. The data support the possibility that the SLC22 and other families of transporters, known to be expressed in many tissues and primarily known for drug and toxin clearance, are integral to a number of endogenous pathways and may be involved in a larger remote sensing and signaling system (Ahn, S. Y., and Nigam, S. K. (2009) Mol. Pharmacol. 76, 481-490, and Wu, W., Dnyanmote, A. V., and Nigam, S. K. (2011) Mol. Pharmacol. 79, 795-805). Drugs may alter metabolism by competing for OAT1 binding of metabolites.
Figures


References
-
- Brenner B. M., Rector F. C. (2004) Brenner & Rector's The Kidney, 7th Ed., pp. 231–415, W. B. Saunders Co., Philadelphia, PA
-
- Eraly S. A., Monte J. C., Nigam S. K. (2004) Physiol. Genomics 18, 12–24 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

