Optical imaging in cancer research: basic principles, tumor detection, and therapeutic monitoring
- PMID: 21757928
- PMCID: PMC7388590
- DOI: 10.1159/000327655
Optical imaging in cancer research: basic principles, tumor detection, and therapeutic monitoring
Abstract
Accurate and rapid detection of diseases is of great importance for assessing the molecular basis of pathogenesis, preventing the onset of complications, and implementing a tailored therapeutic regimen. The ability of optical imaging to transcend wide spatial imaging scales ranging from cells to organ systems has rejuvenated interest in using this technology for medical imaging. Moreover, optical imaging has at its disposal diverse contrast mechanisms for distinguishing normal from pathologic processes and tissues. To accommodate these signaling strategies, an array of imaging techniques has been developed. Importantly, light absorption, and emission methods, as well as hybrid optical imaging approaches are amenable to both small animal and human studies. Typically, complex methods are needed to extract quantitative data from deep tissues. This review focuses on the development of optical imaging platforms, image processing techniques, and molecular probes, as well as their applications in cancer diagnosis, staging, and monitoring therapeutic response.
Copyright © 2011 S. Karger AG, Basel.
Figures

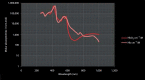




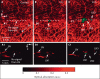

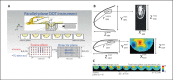


References
-
- Hebden JC, Arridge , SR, Delpy DT. Optical imaging in medicine. I. Experimental techniques. Phys Med Biol. 1997;42:825–840. - PubMed
-
- Sokolov K, Follen M, Richards-Kortum R. Optical spectroscopy for detection of neoplasia. Curr Opin Chem Biol. 2002;6:651–658. - PubMed
-
- Tuchin VV. Tissue optics: light scattering methods and instruments for medical diagnosis. ed 2. Bellingham: SPIE Publications; 2007.
-
- Vo-Dinh T. Biomedical Photonics Handbook. Boca Raton: CRC Press LLC; 2003.
-
- Wang LV. Photoacoustic imaging and spectroscopy. Boca Raton: CRC; 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

