Ecallantide is a novel treatment for attacks of hereditary angioedema due to C1 inhibitor deficiency
- PMID: 21760740
- PMCID: PMC3133501
- DOI: 10.2147/CCID.S10322
Ecallantide is a novel treatment for attacks of hereditary angioedema due to C1 inhibitor deficiency
Abstract
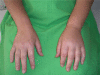
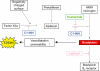
Hereditary angioedema (HAE) resulting from the deficiency of the C1 inhibitor protein is a rare disease, characterized by paroxysms of edema formation in the subcutis and in the submucosa. Edema can cause obstruction of the upper airway, which may lead to suffocation. Prompt elimination of edema is necessary to save patients from this life-threatening condition. Essentially, these edematous attacks are related to the activation of the kinin-kallikrein system and the consequent release of bradykinin. Ecallantide (known as DX-88 previously), a potent and specific inhibitor of plasma kallikrein is an innovative medicinal product. This is the only agent approved recently by the FDA for all localizations of edematous HAE attacks. Its advantages include no risk of viral contamination, high selectivity, very rapid onset of action, good tolerability, and straightforward subcutaneous administration. Owing to the risk of anaphylaxis, ecallantide should be administered by a health care professional. A postmarketing survey to improve risk-assessment and risk-minimization has been launched. The results of these studies may lead to the approval of ecallantide for self-administration.
Keywords: C1-inhibitor deficiency; bradykinin; hereditary angioedema; kallikrein inhibitor; subcutaneous administration; treatment.
Figures
References
-
- Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005;53(3):373–388. 389–392. quiz. - PubMed
-
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy. 2007;62(8):842–856. - PubMed
-
- Donaldson VH, Evans RR. A biochemical abnormality in hereditary angioneurotic edema: absence of serum inhibitor of C’ 1-esterase. Am J Med. 1963;35:37–44. - PubMed
-
- HAEdb, C1 inhibitor gene mutation database. Home page on the Internet. [Updated 2008 July 18]. Available at: http://hae.enzim.hu.
-
- Agostoni A, Cicardi M. Hereditary and acquired C1-inhibitor deficiency: biological and clinical characteristics in 235 patients. Medicine (Baltimore) 1992;71(4):206–215. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

