Seed Oil of Brucea javanica Induces Apoptotic Death of Acute Myeloid Leukemia Cells via Both the Death Receptors and the Mitochondrial-Related Pathways
- PMID: 21760826
- PMCID: PMC3132896
- DOI: 10.1155/2011/965016
Seed Oil of Brucea javanica Induces Apoptotic Death of Acute Myeloid Leukemia Cells via Both the Death Receptors and the Mitochondrial-Related Pathways
Abstract
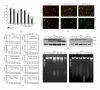
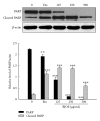
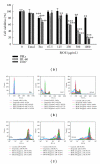
Seed oil of Brucea javanica (BJO) is extracted from the seeds of herb medicine Brucea javanica (L.), and its emulsion formulation (BJOE) has been used clinically to treat carcinomas for many years in China. The antileukemia potential of BJO was investigated in human acute myeloid leukemia cell lines (AML) U937 and HL-60 in vitro and in a mouse U937 xenograft tumor model. BJO induced AML cell apoptosis through activation of caspase-8 and modulation of apoptosis-related proteins. Meanwhile, the inhibition of survivin and XIAP increased the cytotoxicity of BJO. Consistent with these findings, BJO also increased subG(1) phase cells and cause PARP cleavage in AML patients' leukemia cells. In contrast, only weak cytotoxicity of BJO was found in peripheral blood lymphocytes (PBLs) of healthy volunteers. Moreover, oleic acid and linoleic acid were found to be the active components of BJO. Our study provided strong evidence for the first time that BJO induced apoptosis of both cultured and primary AML cells. Furthermore, intravenous injection of BJO significantly inhibited U937 tumor growth in the xenograft mouse model. These results suggest that BJO may have a therapeutic role in the treatment of human leukemia.
Figures






References
-
- Su SY. Treatment of lung cancer with brain metastasis using an intravenous drip of a 10% emulsion of Brucea javanica seminal oil. Chinese Journal of Integrative Medicine. 1985;5(2):86–88. - PubMed
-
- Ma L, Zhang YN. Effects of seminal oil emulsion of Brucea javanica on apoptosis and apoptosis-related genes in human hepatocellular carcinoma cells. World Chinese Journal of Digestology. 2004;12(3):559–562.
-
- He DL, Nan XY, Liu WS. The antitumor effect of 10% Brucea Javanica oil emulsion on prostate cancer cells. Journal of Clinical Urology. 1994;9:60–62.
-
- Li XW, Wang H, Qin WJ, Li X, Liu T, Fan CM. Necrosis and apoptosis induced by Brucea Javanica oil emulsion in bladder cancer cell. Chinese Journal Rehabilitation Theory and Practice. 2004;10(3):163–165.
-
- Li Y, Li Y, Zhang LY, Xu GL. Experimental study of Brucea Javanica oil emulsion induceds K562 cell apoptosis and its mechanism. Journal of International Oncology. 2006;33(8):637–639.
LinkOut - more resources
Full Text Sources

