Systemic activation of K-ras rapidly induces gastric hyperplasia and metaplasia in mice
- PMID: 21761008
- PMCID: PMC3134228
Systemic activation of K-ras rapidly induces gastric hyperplasia and metaplasia in mice
Abstract
Mouse models with conditional activation of K-ras (K-ras(G12D)) are used widely to investigate the role of oncogenic K-ras in a tissue-specific manner. However, the effect of ubiquitous activation of K-ras in adult mice has not been well studied. Herein, we report that systemic activation of K-ras in mice leads to rapid changes in gastric cellular homeostasis. Conditional activation of K-ras results in activation of the MAPK pathway and hyperproliferation of squamous epithelium in the forestomach and metaplasia in the glandular stomach. Parietal cells almost completely disappear from the upper part of the stomach adjacent to forestomach of K-ras activated mice. CDX2, a caudal-related homeobox transcription factor normally expressed in the intestine, is upregulated in parts of the stomach, following activation of K-ras in mice. Cyclooxygenase 2 (COX-2), a mediator of inflammation, is also upregulated in parts of the stomach of the K-ras activated mice with concomitant infiltration of hematopoietic cells in the hyperplastic tissue. Moreover, in K-ras activated mice, the expression of putative progenitor cell marker Dcamkl1 is upregulated in the glandular stomach. Expression of CD44, a candidate stomach cancer stem cell marker, is also increased in forestomach and the glandular stomach. These results suggest that cells of the stomach, potentially stem or progenitor cells, are highly susceptible to K-ras activation-induced initiation of gastric precancerous lesions. The histological changes in the K-ras activated mice resemble the pre-neoplastic changes that take place during gastric carcinogenesis in humans. Thus, a mouse model with systemic K-ras(G12D) activation could be useful for studying the early molecular events leading to gastric carcinogenesis.
Figures



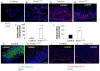




References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Lauren P. The Two Histological Main Types Of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt At A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. - PubMed
-
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. - PubMed
-
- Vojtek AB, Der CJ. Increasing complexity of the Ras signaling pathway. J Biol Chem. 1998;273:19925–8. - PubMed
-
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
