Systemic administration of antisense oligonucleotides simultaneously targeting CK2α and α' subunits reduces orthotopic xenograft prostate tumors in mice
- PMID: 21761204
- PMCID: PMC3893918
- DOI: 10.1007/s11010-011-0943-x
Systemic administration of antisense oligonucleotides simultaneously targeting CK2α and α' subunits reduces orthotopic xenograft prostate tumors in mice
Abstract
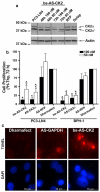
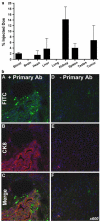
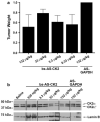
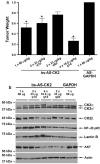
CK2 is a highly conserved, ubiquitous, signal responsive protein serine/threonine kinase. CK2 promotes cell proliferation and suppresses apoptosis, and increased CK2 expression is observed in all cancers examined. We previously reported that direct injection of antisense (AS) CK2α phosphorothioate oligonucleotides (PTO) into xenograft prostate tumors in mice significantly reduced tumor size. Downregulation of CK2α in tumor cells in vivo appeared to result in overexpression of CK2α' protein. This suggested that in cancer cells downregulation of CK2α might be compensated by CK2α' in vivo, prompting us to design a bispecific (bs) AS PTO (bs-AS-CK2) targeting both catalytic subunits. bs-AS-CK2 reduced CK2α and α' protein expression, decreased cell proliferation, and induced apoptosis in cultured cells. Biodistribution studies of administered bs-AS-CK2 oligonucleotide demonstrated its presence in orthotopic prostate xenograft tumors. High dose injections of bs-AS-CK2 resulted in no damage to normal liver or prostate, but induced extensive cell death in tumor tissue. Intraperitoneal treatment with bs-AS-CK2 PTO decreased orthotopic tumor size and downregulated both CK2 mRNA and protein expression. Tumor reduction was accomplished using remarkably low doses and was improved by dividing the dose using a multi-day schedule. Decreased expression of the key signaling pathway proteins NF-κB p65 and AKT was also observed. We propose that the molecular downregulation of CK2 through bispecific targeting of the two catalytic subunits may be uniquely useful for therapeutic elimination of tumors.
Figures


Similar articles
-
Induction of apoptosis by antisense CK2 in human prostate cancer xenograft model.Mol Cancer Res. 2004 Dec;2(12):712-21. Mol Cancer Res. 2004. PMID: 15634760
-
CK2 modulation of NF-kappaB, TP53, and the malignant phenotype in head and neck cancer by anti-CK2 oligonucleotides in vitro or in vivo via sub-50-nm nanocapsules.Clin Cancer Res. 2010 Apr 15;16(8):2295-307. doi: 10.1158/1078-0432.CCR-09-3200. Epub 2010 Apr 6. Clin Cancer Res. 2010. PMID: 20371694 Free PMC article.
-
Impact of protein kinase CK2 downregulation and inhibition on oncomir clusters 17 ~ 92 and 106b ~ 25 in prostate, breast, and head and neck cancers.Mol Med. 2024 Oct 11;30(1):175. doi: 10.1186/s10020-024-00937-1. Mol Med. 2024. PMID: 39394061 Free PMC article.
-
Targeting CK2 for cancer therapy.Anticancer Drugs. 2005 Nov;16(10):1037-43. doi: 10.1097/00001813-200511000-00001. Anticancer Drugs. 2005. PMID: 16222144 Review.
-
[Casein kinase 2, the versatile regulator of cell survival].Mol Biol (Mosk). 2012 May-Jun;46(3):423-33. Mol Biol (Mosk). 2012. PMID: 22888632 Review. Russian.
Cited by
-
Evaluation of protein kinase CK2 as a therapeutic target for squamous cell carcinoma of cats.Am J Vet Res. 2017 Aug;78(8):946-953. doi: 10.2460/ajvr.78.8.946. Am J Vet Res. 2017. PMID: 28738012 Free PMC article.
-
[Protein kinase CK2 and human malignant tumors].Zhongguo Fei Ai Za Zhi. 2012 Jul;15(7):439-45. doi: 10.3779/j.issn.1009-3419.2012.07.09. Zhongguo Fei Ai Za Zhi. 2012. PMID: 22814265 Free PMC article. Review. Chinese. No abstract available.
-
Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: Involvement of multiple signal transduction pathways.Sci Rep. 2016 Mar 15;6:23077. doi: 10.1038/srep23077. Sci Rep. 2016. PMID: 26975752 Free PMC article.
-
CK2 targeted RNAi therapeutic delivered via malignant cell-directed tenfibgen nanocapsule: dose and molecular mechanisms of response in xenograft prostate tumors.Oncotarget. 2016 Sep 20;7(38):61789-61805. doi: 10.18632/oncotarget.11442. Oncotarget. 2016. PMID: 27557516 Free PMC article.
-
Nanoencapsulated anti-CK2 small molecule drug or siRNA specifically targets malignant cancer but not benign cells.Cancer Lett. 2012 Feb 1;315(1):48-58. doi: 10.1016/j.canlet.2011.10.007. Epub 2011 Oct 12. Cancer Lett. 2012. PMID: 22050909 Free PMC article.
References
-
- Guerra B, Issinger OG. Protein kinase CK2 in human diseases. Curr Med Chem. 2008;15(19):1870–1886. doi:10.1007/s00018-009-9148-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

