Unexpected opioid activity profiles of analogues of the novel peptide kappa opioid receptor ligand CJ-15,208
- PMID: 21761566
- PMCID: PMC3667675
- DOI: 10.1002/cmdc.201100113
Unexpected opioid activity profiles of analogues of the novel peptide kappa opioid receptor ligand CJ-15,208
Abstract
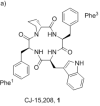
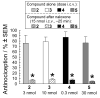
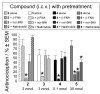
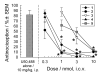
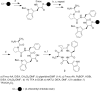
An alanine scan was performed on the novel κ opioid receptor (KOR) peptide ligand CJ-15,208 to determine which residues contribute to the potent in vivo agonist activity observed for the parent peptide. These cyclic tetrapeptides were synthesized by a combination of solid-phase peptide synthesis of the linear precursors, followed by cyclization in solution. Like the parent peptide, each of the analogues exhibited agonist activity and KOR antagonist activity in an antinociceptive assay in vivo. Unlike the parent peptide, the agonist activity of the potent analogues was mediated predominantly, if not exclusively, by μ opioid receptors (MOR). Thus analogues 2 and 4, in which one of the phenylalanine residues was replaced by alanine, exhibited both potent MOR agonist activity and KOR antagonist activity in vivo. These peptides represent novel lead compounds for the development of peptide-based opioid analgesics.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




References
-
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WCJ, Jones RM, Portoghese PS, Carlezon WAJ. J. Pharmacol. Exp. Ther. 2003;305:323–330. - PubMed
- McLaughlin JP, Popovici M. Marton, Chavkin C. J. Neurosci. 2003;23:5674–5683. - PMC - PubMed
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Psychopharmacology (Berl) 2005;183:118–126. - PubMed
-
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr. J. Pharmacol. Exp. Ther. 2007;323:838–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

