Intracellular trafficking of polyamidoamine-poly(ethylene glycol) block copolymers in DNA delivery
- PMID: 21761838
- PMCID: PMC3218102
- DOI: 10.1021/bc200059v
Intracellular trafficking of polyamidoamine-poly(ethylene glycol) block copolymers in DNA delivery
Abstract
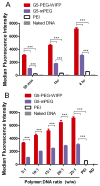
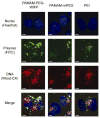
The delivery of nucleic acids has the potential to revolutionize medicine by allowing previously untreatable diseases to be clinically addressed. Viral delivery systems have shown immunogenicity and toxicity dangers, but synthetic vectors have lagged in transfection efficiency. Previously, we developed a modular, linear-dendritic block copolymer architecture with high gene transfection efficiency compared to commercial standards. This rationally designed system makes use of a cationic dendritic block to condense the anionic DNA and forms complexes with favorable endosomal escape properties. The linear block provides biocompatibility and protection from serum proteins, and can be functionalized with a targeting ligand. In this work, we quantitate performance of this system with respect to intracellular barriers to gene delivery using both high-throughput and traditional approaches. An image-based, high-throughput assay for endosomal escape is described and applied to the block copolymer system. Nuclear entry is demonstrated to be the most significant barrier to more efficient delivery and will be addressed in future versions of the system.
Figures



References
-
- Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1:91–99. - PubMed
-
- Schaffert D, Wagner E. Gene therapy progress and prospects: synthetic polymer-based systems. Gene therapy. 2008;15:1131–8. - PubMed
-
- Walther W, Stein U. Viral vectors for gene transfer - A review of their use in the treatment of human diseases. Drugs. 2000;60:249–271. - PubMed
-
- Zaiss AK, Muruve DA. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 2008;15:808–816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

