The NLRP3 inflammasome in health and disease: the good, the bad and the ugly
- PMID: 21762124
- PMCID: PMC3193914
- DOI: 10.1111/j.1365-2249.2011.04440.x
The NLRP3 inflammasome in health and disease: the good, the bad and the ugly
Abstract
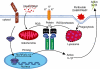
While interleukin (IL)-1β plays an important role in combating the invading pathogen as part of the innate immune response, its dysregulation is responsible for a number of autoinflammatory disorders. Large IL-1β activating platforms, known as inflammasomes, can assemble in response to the detection of endogenous host and pathogen-associated danger molecules. Formation of these protein complexes results in the autocatalysis and activation of caspase-1, which processes precursor IL-1β into its secreted biologically active form. Inflammasome and IL-1β activity is required to efficiently control viral, bacterial and fungal pathogen infections. Conversely, excess IL-1β activity contributes to human disease, and its inhibition has proved therapeutically beneficial in the treatment of a spectrum of serious, yet relatively rare, heritable inflammasomopathies. Recently, inflammasome function has been implicated in more common human conditions, such as gout, type II diabetes and cancer. This raises the possibility that anti-IL-1 therapeutics may have broader applications than anticipated previously, and may be utilized across diverse disease states that are linked insidiously through unwanted or heightened inflammasome activity.
© 2011 The Authors. Clinical and Experimental Immunology © 2011 British Society for Immunology.
Figures

References
-
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. - PubMed
-
- Thornberry NA, Bull HG, Calaycay JR, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–74. - PubMed
-
- Dinarello CA. IL-1: discoveries, controversies and future directions. Eur J Immunol. 2010;40:599–606. - PubMed
-
- Veltri S, Smith JW., II. Interleukin 1 trials in cancer patients: a review of the toxicity, antitumor and hematopoietic effects. Stem Cells. 1996;14:164–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

