Glycyrrhizin as antiviral agent against Hepatitis C Virus
- PMID: 21762538
- PMCID: PMC3169469
- DOI: 10.1186/1479-5876-9-112
Glycyrrhizin as antiviral agent against Hepatitis C Virus
Abstract
Background: Hepatitis C virus is a major cause of chronic liver diseases which can lead to permanent liver damage, hepatocellular carcinoma and death. The presently available treatment with interferon plus ribavirin, has limited benefits due to adverse side effects such as anemia, depression, fatigue, and "flu-like" symptoms. Herbal plants have been used for centuries against different diseases including viral diseases and have become a major source of new compounds to treat bacterial and viral diseases.
Material: The present study was design to study the antiviral effect of Glycyrrhizin (GL) against HCV. For this purpose, HCV infected liver cells were treated with GL at non toxic doses and HCV titer was measured by Quantitative real time RT-PCR.
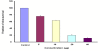
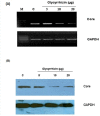
Results and discussion: Our results demonstrated that GL inhibit HCV titer in a dose dependent manner and resulted in 50% reduction of HCV at a concentration of 14 ± 2 μg. Comparative studies were made with interferon alpha to investigate synergistic effects, if any, between antiviral compound and interferon alpha 2a. Our data showed that GL exhibited synergistic effect when combined with interferon. Moreover, these results were verified by transiently transfecting the liver cells with HCV 3a core plasmid. The results proved that GL dose dependently inhibit the expression of HCV 3a core gene both at mRNA and protein levels while the GAPDH remained constant.
Conclusion: Our results suggest that GL inhibit HCV full length viral particles and HCV core gene expression or function in a dose dependent manner and had synergistic effect with interferon. In future, GL along with interferon will be better option to treat HCV infection.
Figures

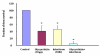
References
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. - DOI - PubMed
-
- Robertson B, Myers G, Howard C, Brettin T, Bukh J, Gaschen B, Gojobori T, Maertens G, Mizokami M, Nainan O. et al.Classification, nomenclature, and database development for hepatitis C virus (HCV) and related viruses: proposals for standardization. International Committee on Virus Taxonomy. Arch Virol. 1998;143:2493–2503. doi: 10.1007/s007050050479. - DOI - PubMed
-
- Clarke B. Molecular virology of hepatitis C virus. J Gen Virol. 1997;78(Pt 10):2397–2410. - PubMed
-
- Reed KE, Rice CM. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Curr Top Microbiol Immunol. 2000;242:55–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

