Effectiveness of seasonal influenza vaccine against pandemic (H1N1) 2009 virus, Australia, 2010
- PMID: 21762570
- PMCID: PMC3381383
- DOI: 10.3201/eid1707.101959
Effectiveness of seasonal influenza vaccine against pandemic (H1N1) 2009 virus, Australia, 2010
Abstract
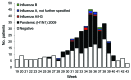
To estimate effectiveness of seasonal trivalent and monovalent influenza vaccines against pandemic influenza A (H1N1) 2009 virus, we conducted a test-negative case-control study in Victoria, Australia, in 2010. Patients seen for influenza-like illness by general practitioners in a sentinel surveillance network during 2010 were tested for influenza; vaccination status was recorded. Case-patients had positive PCRs for pandemic (H1N1) 2009 virus, and controls had negative influenza test results. Of 319 eligible patients, test results for 139 (44%) were pandemic (H1N1) 2009 virus positive. Adjusted effectiveness of seasonal vaccine against pandemic (H1N1) 2009 virus was 79% (95% confidence interval 33%-93%); effectiveness of monovalent vaccine was 47% and not statistically significant. Vaccine effectiveness was higher among adults. Despite some limitations, this study indicates that the first seasonal trivalent influenza vaccine to include the pandemic (H1N1) 2009 virus strain provided significant protection against laboratory-confirmed pandemic (H1N1) 2009 infection.
Figures
References
-
- World Health Organization. World now at the start of 2009 influenza pandemic. 2009. Jun 11 [cited 2010 Oct 29]. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6...
-
- World Health Organization. Availability of a candidate reassortant vaccine virus for the novel influenza A (H1N1) vaccine development. 2009. Jun 4 [cited 2010 Oct 29]. http://www.who.int/csr/resources/publications/swineflu/ivr153_20090608_e...
-
- Australian Government Department of Health and Ageing. Get vaccinated. 2009. [cited 2010 Oct 29]. http://www.flupandemic.gov.au/internet/panflu/publishing.nsf/Content/H1N...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical