The PepT1-NOD2 signaling pathway aggravates induced colitis in mice
- PMID: 21762661
- PMCID: PMC3186842
- DOI: 10.1053/j.gastro.2011.06.080
The PepT1-NOD2 signaling pathway aggravates induced colitis in mice
Abstract
Background & aims: The human di/tripeptide transporter human intestinal H-coupled oligonucleotide transporter (hPepT1) is abnormally expressed in colons of patients with inflammatory bowel disease, although its exact role in pathogenesis is unclear. We investigated the contribution of PepT1 to intestinal inflammation in mouse models of colitis and the involvement of the nucleotide-binding oligomerization domain 2 (NOD2) signaling pathway in the pathogenic activity of colonic epithelial hPepT1.
Methods: Transgenic mice were generated in which hPepT1 expression was regulated by the β-actin or villin promoters; colitis was induced using 2,4,6-trinitrobenzene sulfonic acid (TNBS) or dextran sodium sulfate (DSS) and the inflammatory responses were assessed. The effects of NOD2 deletion in the hPepT1 transgenic mice also was studied to determine the involvement of the PepT1-NOD2 signaling pathway.
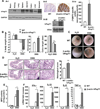
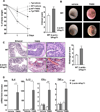
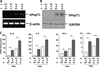
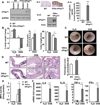
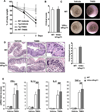
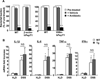
Results: TNBS and DSS induced more severe levels of inflammation in β-actin-hPepT1 transgenic mice than wild-type littermates. Intestinal epithelial cell-specific hPepT1 overexpression in villin-hPepT1 transgenic mice increased the severity of inflammation induced by DSS, but not TNBS. Bone marrow transplantation studies showed that hPepT1 expression in intestinal epithelial cells and immune cells has an important role in the proinflammatory response. Antibiotics abolished the effect of hPepT1 overexpression on the inflammatory response in DSS-induced colitis in β-actin-hPepT1 and villin-hPepT1 transgenic mice, indicating that commensal bacteria are required to aggravate intestinal inflammation. Nod2-/-, β-actin-hPepT1 transgenic/Nod2-/-, and villin-hPepT1 transgenic/Nod2-/- littermates had similar levels of susceptibility to DSS-induced colitis, indicating that hPepT1 overexpression increased intestinal inflammation in a NOD2-dependent manner.
Conclusions: The PepT1-NOD2 signaling pathway is involved in aggravation of DSS-induced colitis in mice.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
No conflicts of interest exists.
Figures

Similar articles
-
Colonic expression of the peptide transporter PEPT1 is downregulated during intestinal inflammation and is not required for NOD2-dependent immune activation.Inflamm Bowel Dis. 2014 Apr;20(4):671-84. doi: 10.1097/01.MIB.0000443336.71488.08. Inflamm Bowel Dis. 2014. PMID: 24583477
-
PepT1 expressed in immune cells has an important role in promoting the immune response during experimentally induced colitis.Lab Invest. 2013 Aug;93(8):888-99. doi: 10.1038/labinvest.2013.77. Epub 2013 Jun 24. Lab Invest. 2013. PMID: 23797361
-
Pathogenic bacteria induce colonic PepT1 expression: an implication in host defense response.Gastroenterology. 2009 Oct;137(4):1435-47.e1-2. doi: 10.1053/j.gastro.2009.06.043. Epub 2009 Jun 21. Gastroenterology. 2009. PMID: 19549526 Free PMC article.
-
Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2.Gastroenterology. 2013 Dec;145(6):1392-403.e1-8. doi: 10.1053/j.gastro.2013.08.033. Epub 2013 Aug 21. Gastroenterology. 2013. PMID: 23973922
-
Enteric Delivery of Regenerating Family Member 3 alpha Alters the Intestinal Microbiota and Controls Inflammation in Mice With Colitis.Gastroenterology. 2018 Mar;154(4):1009-1023.e14. doi: 10.1053/j.gastro.2017.11.003. Epub 2017 Nov 11. Gastroenterology. 2018. PMID: 29133078
Cited by
-
Nod2 deficiency is associated with an increased mucosal immunoregulatory response to commensal microorganisms.Mucosal Immunol. 2014 Mar;7(2):391-404. doi: 10.1038/mi.2013.58. Epub 2013 Aug 21. Mucosal Immunol. 2014. PMID: 23962873 Free PMC article.
-
Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications.Mol Aspects Med. 2013 Apr-Jun;34(2-3):323-36. doi: 10.1016/j.mam.2012.11.003. Mol Aspects Med. 2013. PMID: 23506874 Free PMC article. Review.
-
MicroRNA-193a-3p Reduces Intestinal Inflammation in Response to Microbiota via Down-regulation of Colonic PepT1.J Biol Chem. 2015 Jun 26;290(26):16099-115. doi: 10.1074/jbc.M115.659318. Epub 2015 Apr 30. J Biol Chem. 2015. PMID: 25931122 Free PMC article.
-
Adaptation of adherent-invasive E. coli to gut environment: Impact on flagellum expression and bacterial colonization ability.Gut Microbes. 2020 May 3;11(3):364-380. doi: 10.1080/19490976.2017.1421886. Epub 2018 Mar 1. Gut Microbes. 2020. PMID: 29494278 Free PMC article.
-
Critical role of PepT1 in promoting colitis-associated cancer and therapeutic benefits of the anti-inflammatory PepT1-mediated tripeptide KPV in a murine model.Cell Mol Gastroenterol Hepatol. 2016 May;2(3):340-357. doi: 10.1016/j.jcmgh.2016.01.006. Cell Mol Gastroenterol Hepatol. 2016. PMID: 27458604 Free PMC article.
References
-
- Merlin D, Si-Tahar M, Sitaraman SV, et al. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology. 2001;120:1666–1679. - PubMed
-
- Wojtal KA, Eloranta JJ, Hruz P, et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos. 2009;37:1871–1877. - PubMed
-
- Mathews DM, Adibi SA. Peptide absorption. Gastroenterology. 1976;71:151–161. - PubMed
-
- Adibi SA. Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol Gastrointest Liver Physiol. 2003;285:G779–G788. - PubMed
-
- Fei YJ, Kanai Y, Nussberger S, et al. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

