Spiral ganglion neuron survival and function in the deafened cochlea following chronic neurotrophic treatment
- PMID: 21762764
- PMCID: PMC3205216
- DOI: 10.1016/j.heares.2011.06.007
Spiral ganglion neuron survival and function in the deafened cochlea following chronic neurotrophic treatment
Abstract
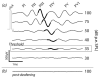
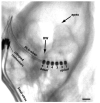
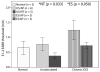
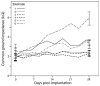
Cochlear implants electrically stimulate residual spiral ganglion neurons (SGNs) to provide auditory cues for the severe-profoundly deaf. However, SGNs gradually degenerate following cochlear hair cell loss, leaving fewer neurons available for stimulation. Providing an exogenous supply of neurotrophins (NTs) has been shown to prevent SGN degeneration, and when combined with chronic intracochlear electrical stimulation (ES) following a short period of deafness (5 days), may also promote the formation of new neurons. The present study assessed the histopathological response of guinea pig cochleae treated with NTs (brain-derived neurotrophic factor and neurotrophin-3) with and without ES over a four week period, initiated two weeks after deafening. Results were compared to both NT alone and artificial perilymph (AP) treated animals. AP/ES treated animals exhibited no evidence of SGN rescue compared with untreated deafened controls. In contrast, NT administration showed a significant SGN rescue effect in the lower and middle cochlear turns (two-way ANOVA, p < 0.05) compared with AP-treated control animals. ES in combination with NT did not enhance SGN survival compared with NT alone. SGN function was assessed by measuring electrically-evoked auditory brainstem response (EABR) thresholds. EABR thresholds following NT treatment were significantly lower than animals treated with AP (two-way ANOVA, p = 0.033). Finally, the potential for induced neurogenesis following the combined treatment was investigated using a marker of DNA synthesis. However, no evidence of neurogenesis was observed in the SGN population. The results indicate that chronic NT delivery to the cochlea may be beneficial to cochlear implant patients by increasing the number of viable SGNs and decreasing activation thresholds compared to chronic ES alone.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures



References
-
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. - PubMed
-
- Agterberg MJ, Versnel H, de Groot JC, Smoorenburg GF, Albers FW, Klis SF. Morphological changes in spiral ganglion cells after intracochlear application of brain-derived neurotrophic factor in deafened guinea pigs. Hear Res. 2008;244:25–34. - PubMed
-
- Agterberg MJ, Versnel H, de Groot JC, van den Broek M, Klis SF. Chronic electrical stimulation does not prevent spiral ganglion cell degeneration in deafened guinea pigs. Hear Res. 2010;269:169–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

